Neurophysiological biomarkers for drug development in schizophrenia
- PMID: 18064038
- PMCID: PMC2753449
- DOI: 10.1038/nrd2463
Neurophysiological biomarkers for drug development in schizophrenia
Abstract
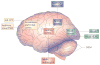
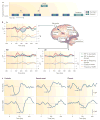
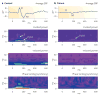
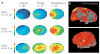
Schizophrenia represents a pervasive deficit in brain function, leading to hallucinations and delusions, social withdrawal and a decline in cognitive performance. As the underlying genetic and neuronal abnormalities in schizophrenia are largely unknown, it is challenging to measure the severity of its symptoms objectively, or to design and evaluate psychotherapeutic interventions. Recent advances in neurophysiological techniques provide new opportunities to measure abnormal brain functions in patients with schizophrenia and to compare these with drug-induced alterations. Moreover, many of these neurophysiological processes are phylogenetically conserved and can be modelled in preclinical studies, offering unique opportunities for use as translational biomarkers in schizophrenia drug discovery.
Figures

References
-
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. Reviews evidence for gamma-band synchrony in humans and its possible roles, as well as methods for non-invasive measurement of neural synchrony. - PubMed
-
- Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamics of human object recognition processing: an integrated high-density electrical mapping and functional imaging study of “closure” processes. Neuroimage. 2006;29:605–618. - PubMed
-
- Swerdlow NR, et al. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

