Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease
- PMID: 18064301
- PMCID: PMC2117760
- DOI: 10.1172/JCI30989
Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease
Abstract
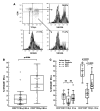
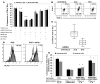
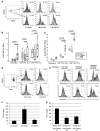
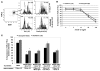
Intraepithelial lymphocytes (IELs) bearing the gammadelta TCR are more abundant in the small intestinal mucosa of patients with celiac disease (CD) compared with healthy individuals. However, their role in disease pathogenesis is not well understood. Here, we investigated the functional attributes of TCRgammadelta+ IELs isolated from intestinal biopsies of patients with either active celiac disease (ACD) or those on a gluten-free diet (GFD). We found that compared with individuals with ACD, individuals on GFD have a higher frequency of CD8+TCRgammadelta+ IELs that express the inhibitory NK receptor NKG2A and intracellular TGF-beta1. TCR triggering as well as cross-linking of NKG2A increased both TGF-beta1 intracellular expression and secretion in vitro. Coculture of sorted TCRgammadelta+NKG2A+ IELs, IL-15-stimulated TCRalphabeta+ IELs, and HLA-E+ enterocytes resulted in a decreased percentage of cytotoxic CD8+TCRalphabeta+ IELs expressing intracellular IFN-gamma and granzyme-B and surface NKG2D. This inhibition was partially abrogated by blocking either TGF-beta alone or both NKG2A and HLA-E. Thus, our data indicate that suppression was at least partially mediated by TGF-beta secretion as a result of engagement of NKG2A with its ligand, HLA-E, on enterocytes and/or TCRalphabeta+ IELs. These findings demonstrate that human small intestinal CD8+TCRgammadelta+ IELs may have regulatory potential in celiac disease.
Figures


References
-
- Green P.H., Jabri B. Celiac disease. Annu. Rev. Med. 2006;57:207–221. - PubMed
-
- Shan L., et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. - PubMed
-
- Gianfrani C., et al. Celiac disease association with CD8+ T cell responses: identification of a novel gliadin-derived HLA-A2-restricted epitope. . J. Immunol. 2003;170:2719–2726. - PubMed
-
- Meresse B., et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. - PubMed
-
- Hue S., et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

