Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells
- PMID: 18064302
- PMCID: PMC2117762
- DOI: 10.1172/JCI32040
Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells
Abstract
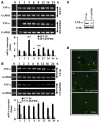
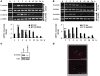
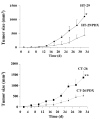
The proprotein convertases (PCs) are implicated in the activation of various precursor proteins that play an important role in tumor cell metastasis. Here, we report their involvement in the regulation of the metastatic potential of colorectal tumor cells. PC function in the human and murine colon carcinoma cell lines HT-29 and CT-26, respectively, was inhibited using siRNA targeting the PCs furin, PACE4, PC5, and PC7 or by overexpression of the general PC inhibitor alpha1-antitrypsin Portland (alpha1-PDX). We found that overexpression of alpha1-PDX and knockdown of furin expression inhibited processing of IGF-1 receptor and its subsequent activation by IGF-1 to induce IRS-1 and Akt phosphorylation, all important in colon carcinoma metastasis. These data suggest that the PC furin is a major IGF-1 receptor convertase. Expression of alpha1-PDX reduced the production of TNF-alpha and IL-1alpha by human colon carcinoma cells, and incubation of murine liver endothelial cells with conditioned media derived from these cells failed to induce tumor cell adhesion to activated murine endothelial cells, a critical step in metastatic invasion. Furthermore, colon carcinoma cells in which PC activity was inhibited by overexpression of alpha1-PDX when injected into the portal vein of mice showed a significantly reduced ability to form liver metastases. This suggests that inhibition of PCs is a potentially promising strategy for the prevention of colorectal liver metastasis.
Figures
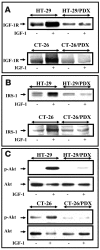
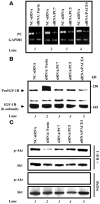
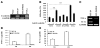


References
-
- Choti M.A., Bulkley G.B. Management of hepatic metastases. Liver Transpl. Surg. 1999;5:65–80. - PubMed
-
- Hiratsuka M., et al. Management of patients with hepatic metastases from gastric carcinoma. Nippon Geka Gakkai Zasshi. 2003;104:711–716. - PubMed
-
- Jaeck D. The significance of hepatic pedicle lymph nodes metastases in surgical management of colorectal liver metastases and of other liver malignancies. Ann. Surg. Oncol. 2003;10:1007–1011. - PubMed
-
- Vallet C., Martinet O., Mosimann F. Surgical treatment of hepatic metastases. Rev. Med. Suisse Romande. 2001;121:119–124. - PubMed
-
- Kitakata H., et al. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res. 2002;62:6682–6687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

