Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells
- PMID: 18064304
- PMCID: PMC2117766
- DOI: 10.1172/JCI32412
Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells
Abstract
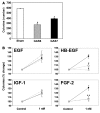
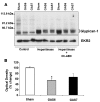
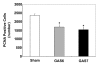
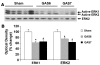
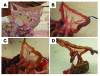
Cells isolated from many types of human cancers express heparin-binding growth factors (HBGFs) that drive tumor growth, metastasis, and angiogenesis. The heparan sulfate proteoglycan glypican-1 (GPC1) is a coreceptor for HBGFs. Here we show that both cancer cell-derived and host-derived GPC1 are crucial for efficient growth, metastasis, and angiogenesis of human and mouse cancer cells. Thus downregulation of GPC1 in the human pancreatic cancer cell line PANC-1, using antisense approaches, resulted in prolonged doubling times and decreased anchorage-independent growth in vitro as well as attenuated tumor growth, angiogenesis, and metastasis when these cells were transplanted into athymic mice. Moreover, athymic mice that lacked GPC1 exhibited decreased tumor angiogenesis and metastasis following intrapancreatic implantation with either PANC-1 or T3M4 human pancreatic cancer cells and fewer pulmonary metastases following intravenous injection of murine B16-F10 melanoma cells. In addition, hepatic endothelial cells isolated from these mice exhibited an attenuated mitogenic response to VEGF-A. These data indicate that cancer cell- and host-derived GPC1 are crucial for full mitogenic, angiogenic, and metastatic potential of cancer cells. Thus targeting GPC1 might provide new avenues for cancer therapy and for the prevention of cancer metastasis.
Figures







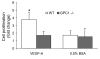

References
-
- McKenna S., Eatock M. The medical management of pancreatic cancer: a review. Oncologist. 2003;8:149–160. - PubMed
-
- Jemal A., et al. Cancer statistics. CA Cancer J. Clin. 2007;57:43–66. - PubMed
-
- Perrimon N., Bernfield M. Cellular functions of proteoglycans — an overview. Semin. Cell Dev. Biol. 2001;12:65–67. - PubMed
-
- De Cat B., David G. Developmental roles of the glypicans. Semin. Cell Dev. Biol. 2001;12:117–125. - PubMed
-
- Filmus J. Glypicans in growth control and cancer. Glycobiology. 2001;11:19R–23R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

