Multiphoton imaging can be used for microscopic examination of intact human gastrointestinal mucosa ex vivo
- PMID: 18065276
- PMCID: PMC2254558
- DOI: 10.1016/j.cgh.2007.10.008
Multiphoton imaging can be used for microscopic examination of intact human gastrointestinal mucosa ex vivo
Abstract
Background & aims: The ability to observe cellular and subcellular detail during routine endoscopy is a major goal in the development of new endoscopic imaging techniques. Multiphoton microscopy, which relies on nonlinear infrared optical processes, has the potential to identify cellular details by excitation of endogenous fluorescent molecules. We examined the feasibility of using multiphoton microscopy to characterize mucosal histology in the human gastrointestinal tract.
Methods: A multiphoton microscope was used to determine the optimal excitation wavelength for examination of gastrointestinal mucosa. Fresh, unfixed, and unstained biopsy specimens obtained during routine endoscopy in human subjects then were examined by confocal microscopy and multiphoton microscopy. Multiphoton images also were compared with standard H&E images obtained from paired biopsy specimens. A prototype miniaturized multiphoton probe was used to examine intact rat colon.
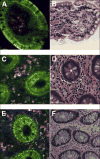
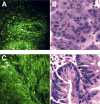
Results: Peak multiphoton autofluorescence intensity was detected in mucosa excited at 735 nm. Multiphoton microscopic examination of unstained biopsy specimens revealed improved cellular detail relative to either unstained or stained specimens examined by confocal imaging. Resolution of structures such as epithelial nuclei, goblet cells, and interstitial fibers and cells was comparable with what was obtained using standard H&E histology. Similar findings were observed when using a prototype miniaturized multiphoton probe.
Conclusions: Multiphoton microscopy can be used to examine gastrointestinal mucosa at the cellular level, without the need for fluorescent dyes. The construction of a multiphoton endomicroscope therefore could provide a practical means of performing virtual biopsies during the course of routine endoscopy, with advantages over currently available endomicroscopy technologies.
Figures





References
-
- Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275–1283. - PubMed
-
- Norfleet R, Ryan M, Wyman J. Adenomatous and hyperplastic polyps cannot be reliably distinguished by their appearance through the fiberoptic sigmoidscope. Dig Dis Sci. 1988;33:1175–1177. - PubMed
-
- Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, Nafe B, Galle PR, Neurath MF. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. - PubMed
-
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. - PubMed
-
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

