Ligand-stabilized conformational states of human beta(2) adrenergic receptor: insight into G-protein-coupled receptor activation
- PMID: 18065472
- PMCID: PMC2257890
- DOI: 10.1529/biophysj.107.117648
Ligand-stabilized conformational states of human beta(2) adrenergic receptor: insight into G-protein-coupled receptor activation
Abstract
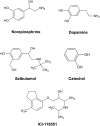
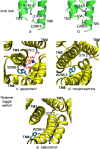
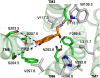
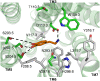
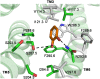
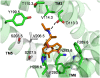
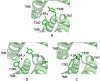
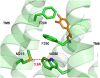
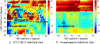
G-protein-coupled receptors (GPCRs) are known to exist in dynamic equilibrium between inactive- and several active-state conformations, even in the absence of a ligand. Recent experimental studies on the beta(2) adrenergic receptor (beta(2)AR) indicate that structurally different ligands with varying efficacies trigger distinct conformational changes and stabilize different receptor conformations. We have developed a computational method to study the ligand-induced rotational orientation changes in the transmembrane helices of GPCRs. This method involves a systematic spanning of the rotational orientation of the transmembrane helices (TMs) that are in the vicinity of the ligand for predicting the helical rotations that occur on ligand binding. The predicted ligand-stabilized receptor conformations are characterized by a simultaneous lowering of the ligand binding energy and a significant gain in interhelical and receptor-ligand hydrogen bonds. Using the beta(2)AR as a model, we show that the receptor conformational state depends on the structure and efficacy of the ligand for a given signaling pathway. We have studied the ligand-stabilized receptor conformations of five different ligands, a full agonist, norepinephrine; a partial agonist, salbutamol; a weak partial agonist, dopamine; a very weak agonist, catechol; and an inverse agonist, ICI-115881. The predicted ligand-stabilized receptor models correlate well with the experimentally observed conformational switches in beta(2)AR, namely, the breaking of the ionic lock between R131(3.50) at the intracellular end of TM3 (part of the DRY motif) and E268(6.30) on TM6, and the rotamer toggle switch on W286(6.48) on TM6. In agreement with trp-bimane quenching experiments, we found that norepinephrine and dopamine break the ionic lock and engage the rotamer toggle switch, whereas salbutamol, a noncatechol partial agonist only breaks the ionic lock, and the weak agonist catechol only engages the rotamer toggle switch. Norepinephrine and dopamine occupy the same binding region, between TM3, TM5, and TM6, whereas the binding site of salbutamol is shifted toward TM4. Catechol binds deeper into the protein cavity compared to the other ligands, making contact with TM5 and TM6. A part of the catechol binding site overlaps with those of dopamine and norepinephrine but not with that of salbutamol. Virtual ligand screening on 10,060 ligands on the norepinephrine-stabilized receptor conformation shows an enrichment of 38% compared to ligand unbound receptor conformation. These results show that ligand-induced conformational changes are important for developing functionally specific drugs that will stabilize a particular receptor conformation. These studies represent the first step toward a more universally applicable computational method for studying ligand efficacy and GPCR activation.
Figures

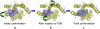
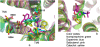
References
-
- Hubbell, W. L., C. Altenbach, C. M. Hubbell, and H. G. Khorana. 2003. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv. Protein Chem. 63:243–290. - PubMed
-
- Bartfai, T., J. Benovic, J. Bockaert, R. A. Bond, M. Bouvier, A. Christopoulos, O. Civelli, L. A. Devi, S. R. George, A. Inui, B. K. Kobilka, R. Leurs, R. Neubig, J.-P. Pin, R. Quirion, B. P. Roques, T. P. Sakmar, R. Seifert, R. E. Stenkamp, and P. G. Strange. 2004. The state of GPCR research in 2004. Nat. Rev. Drug Discov. 3:577–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

