CotC-CotU heterodimerization during assembly of the Bacillus subtilis spore coat
- PMID: 18065538
- PMCID: PMC2238189
- DOI: 10.1128/JB.01425-07
CotC-CotU heterodimerization during assembly of the Bacillus subtilis spore coat
Abstract
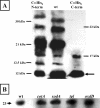
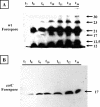
We report evidence that CotC and CotU, two previously identified components of the Bacillus subtilis spore coat, are produced concurrently in the mother cell chamber of the sporulating cell under the control of sigmaK and GerE and immediately assembled around the forming spore. In the coat, the two proteins interact to form a coat component of 23 kDa. The CotU-CotC interaction was not detected in two heterologous hosts, suggesting that it occurs only in B. subtilis. Monomeric forms of both CotU and CotC failed to be assembled at the surface of the developing spore and accumulated in the mother cell compartment of cells mutant for cotE. In contrast, neither CotU nor CotC accumulated in the mother cell compartment of cells mutant for cotH. These results suggest that CotH is required to protect both CotU and CotC in the mother cell compartment of the sporangium and that CotE is needed to allow their assembly and subsequent interaction at the spore surface.
Figures



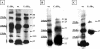

Similar articles
-
CotE binds to CotC and CotU and mediates their interaction during spore coat formation in Bacillus subtilis.J Bacteriol. 2010 Feb;192(4):949-54. doi: 10.1128/JB.01408-09. Epub 2009 Dec 18. J Bacteriol. 2010. PMID: 20023017 Free PMC article.
-
GerE-independent expression of cotH leads to CotC accumulation in the mother cell compartment during Bacillus subtilis sporulation.Microbiology (Reading). 2004 Oct;150(Pt 10):3441-9. doi: 10.1099/mic.0.27356-0. Microbiology (Reading). 2004. PMID: 15470121
-
Assembly of multiple CotC forms into the Bacillus subtilis spore coat.J Bacteriol. 2004 Feb;186(4):1129-35. doi: 10.1128/JB.186.4.1129-1135.2004. J Bacteriol. 2004. PMID: 14762008 Free PMC article.
-
Assembly and genetics of spore protective structures.Cell Mol Life Sci. 2002 Mar;59(3):434-44. doi: 10.1007/s00018-002-8436-4. Cell Mol Life Sci. 2002. PMID: 11964122 Free PMC article. Review.
-
[Identification and characterization of the outermost layer of Bacillus subtilis spores].Yakugaku Zasshi. 2012;132(8):919-24. doi: 10.1248/yakushi.132.919. Yakugaku Zasshi. 2012. PMID: 22864350 Review. Japanese.
Cited by
-
Mucosal adjuvant activity of IL-2 presenting spores of bacillus subtilis in a murine model of Helicobacter pylori vaccination.PLoS One. 2014 Apr 17;9(4):e95187. doi: 10.1371/journal.pone.0095187. eCollection 2014. PLoS One. 2014. PMID: 24743850 Free PMC article.
-
Progress in research and application development of surface display technology using Bacillus subtilis spores.Appl Microbiol Biotechnol. 2020 Mar;104(6):2319-2331. doi: 10.1007/s00253-020-10348-x. Epub 2020 Jan 27. Appl Microbiol Biotechnol. 2020. PMID: 31989224 Free PMC article. Review.
-
Localization of a red fluorescence protein adsorbed on wild type and mutant spores of Bacillus subtilis.Microb Cell Fact. 2016 Sep 8;15(1):153. doi: 10.1186/s12934-016-0551-2. Microb Cell Fact. 2016. PMID: 27609116 Free PMC article.
-
Antagonistic role of CotG and CotH on spore germination and coat formation in Bacillus subtilis.PLoS One. 2014 Aug 12;9(8):e104900. doi: 10.1371/journal.pone.0104900. eCollection 2014. PLoS One. 2014. PMID: 25115591 Free PMC article.
-
CotG-Like Modular Proteins Are Common among Spore-Forming Bacilli.J Bacteriol. 2016 Apr 28;198(10):1513-20. doi: 10.1128/JB.00023-16. Print 2016 May 15. J Bacteriol. 2016. PMID: 26953338 Free PMC article.
References
-
- Aronson, A. I., H.-Y. Song, and N. Bourne. 1988. Gene structure and precursor processing of a novel Bacillus subtilis spore coat protein. Mol. Microbiol. 3437-444. - PubMed
-
- Baccigalupi, L., G. Castaldo, G. Cangiano, R. Isticato, R. Marasco, M. De Felice, and E. Ricca. 2004. GerE-independent expression of cotH leads to CotC accumulation in the mother cell compartment during Bacillus subtilis sporulation. Microbiology 1503441-3449. - PubMed
-
- Cutting, S., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. Harwood and S. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

