The contribution of photosynthesis to the red light response of stomatal conductance
- PMID: 18065555
- PMCID: PMC2245822
- DOI: 10.1104/pp.107.110924
The contribution of photosynthesis to the red light response of stomatal conductance
Abstract
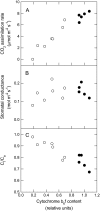
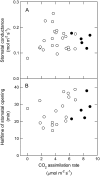
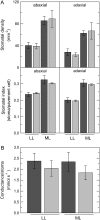
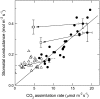
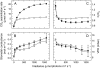
To determine the contribution of photosynthesis on stomatal conductance, we contrasted the stomatal red light response of wild-type tobacco (Nicotiana tabacum 'W38') with that of plants impaired in photosynthesis by antisense reductions in the content of either cytochrome b(6)f complex (anti-b/f plants) or Rubisco (anti-SSU plants). Both transgenic genotypes showed a lowered content of the antisense target proteins in guard cells as well as in the mesophyll. In the anti-b/f plants, CO(2) assimilation rates were proportional to leaf cytochrome b(6)f content, but there was little effect on stomatal conductance and the rate of stomatal opening. To compare the relationship between photosynthesis and stomatal conductance, wild-type plants and anti-SSU plants were grown at 30 and 300 micromol photon m(-2) s(-1) irradiance (low light and medium light [ML], respectively). Growth in ML increased CO(2) assimilation rates and stomatal conductance in both genotypes. Despite the significantly lower CO(2) assimilation rate in the anti-SSU plants, the differences in stomatal conductance between the genotypes were nonsignificant at either growth irradiance. Irrespective of plant genotype, stomatal density in the two leaf surfaces was 2-fold higher in ML-grown plants than in low-light-grown plants and conductance normalized to stomatal density was unaffected by growth irradiance. We conclude that the red light response of stomatal conductance is independent of the concurrent photosynthetic rate of the guard cells or of that of the underlying mesophyll. Furthermore, we suggest that the correlation of photosynthetic capacity and stomatal conductance observed under different light environments is caused by signals largely independent of photosynthesis.
Figures



References
-
- Ball J, Woodrow I, Berry J (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In J Biggins, ed, Progress in Photosynthesis Research, Vol IV. Martinus Nijhoff, Leiden, The Netherlands, pp 221–224
-
- Bergmann D (2006) Stomatal development: from neighbourly to global communication. Curr Opin Plant Biol 9 478–483 - PubMed
-
- Buckley TN, Mott KA, Farquhar GD (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26 1767–1785
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

