Structural basis for the coevolution of a viral RNA-protein complex
- PMID: 18066080
- PMCID: PMC3152963
- DOI: 10.1038/nsmb1327
Structural basis for the coevolution of a viral RNA-protein complex
Abstract
The cocrystal structure of the PP7 bacteriophage coat protein in complex with its translational operator identifies a distinct mode of sequence-specific RNA recognition when compared to the well-characterized MS2 coat protein-RNA complex. The structure reveals the molecular basis of the PP7 coat protein's ability to selectively bind its cognate RNA, and it demonstrates that the conserved beta-sheet surface is a flexible architecture that can evolve to recognize diverse RNA hairpins.
Figures
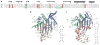

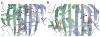
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

