CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides
- PMID: 18067555
- PMCID: PMC2434387
- DOI: 10.1111/j.1365-2567.2007.02749.x
CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides
Abstract
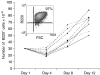
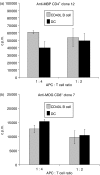
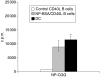
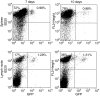
Dendritic cells (DC) are increasingly exploited for cell-based immunotherapy. However, limitations in ex vivo DC growth and DC functional heterogeneity have motivated development of complementary antigen-presenting cell sources. Here, the ability of CD40 ligand (CD40L)-activated B cells to fulfil that role was investigated. We demonstrate for the first time that non-specific or antigen-specific murine B cells can be grown for extended periods of time by stimulation with CD40L. These cells rapidly up-regulate and maintain high levels of co-stimulatory molecules. In a head-to-head comparison with DC, CD40L-expanded B cells were comparable to DC in the presentation of peptides to CD4+ and CD8+ T cells. While DC were superior to antigen non-specific CD40L-activated B cells with regard to whole protein (NP-BSA) processing and presentation, CD40L-expanded B cells from NP-BSA-immunized mice were comparable to DC when presenting BSA or NP-BSA to primed primary T cells or when presenting NP linked to an unrelated carrier, CGG, to naïve T cells. Thus, the combination of CD40L activation, which supports B-cell growth and augments intracellular protein processing, and antigen uptake via the B-cell receptor, allows for efficient uptake, processing, and presentation of whole protein antigens in a fashion comparable to that observed with mature DC. Like DC, CD40L-activated B cells efficiently home to secondary lymphoid organs in vivo. This system represents a unique tool for studying primary antigen-specific B cells and the results suggest that the outgrowth of large numbers of highly activated B cells represents a viable and practical complement to DC for cell-based immunotherapy.
Figures



References
-
- Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. - PubMed
-
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–8. - PubMed
-
- Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–4. - PubMed
-
- Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

