EspFU, a type III-translocated effector of actin assembly, fosters epithelial association and late-stage intestinal colonization by E. coli O157:H7
- PMID: 18067584
- PMCID: PMC2504705
- DOI: 10.1111/j.1462-5822.2007.01087.x
EspFU, a type III-translocated effector of actin assembly, fosters epithelial association and late-stage intestinal colonization by E. coli O157:H7
Abstract
Enterohaemorrhagic Escherichia coli (EHEC) O157:H7 induces filamentous actin-rich 'pedestals' on intestinal epithelial cells. Pedestal formation in vitro requires translocation of bacterial effectors into the host cell, including Tir, an EHEC receptor, and EspF(U), which increases the efficiency of actin assembly initiated by Tir. While inactivation of espF(U) does not alter colonization in two reservoir hosts, we utilized two disease models to explore the significance of EspF(U)-promoted actin pedestal formation. EHECDeltaespF(U) efficiently colonized the rabbit intestine during co-infection with wild-type EHEC, but co-infection studies on cultured cells suggested that EspF(U) produced by wild-type bacteria might have rescued the mutant. Significantly, EHECDeltaespF(U) by itself was fully capable of establishing colonization at 2 days post inoculation but unlike wild type, failed to expand in numbers in the caecum and colon by 7 days. In the gnotobiotic piglet model, an espF(U) deletion mutant appeared to generate actin pedestals with lower efficiency than wild type. Furthermore, aggregates of the mutant occupied a significantly smaller area of the intestinal epithelial surface than those of the wild type. Together, these findings suggest that, after initial EHEC colonization of the intestinal surface, EspF(U) may stabilize bacterial association with the epithelial cytoskeleton and promote expansion beyond initial sites of infection.
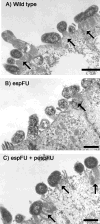
Figures



Similar articles
-
Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation.PLoS Pathog. 2010 Aug 19;6(8):e1001056. doi: 10.1371/journal.ppat.1001056. PLoS Pathog. 2010. PMID: 20808845 Free PMC article.
-
Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6754-9. doi: 10.1073/pnas.0809131106. Epub 2009 Apr 6. Proc Natl Acad Sci U S A. 2009. PMID: 19366662 Free PMC article.
-
Enterohaemorrhagic Escherichia coli Tir requires a C-terminal 12-residue peptide to initiate EspF-mediated actin assembly and harbours N-terminal sequences that influence pedestal length.Cell Microbiol. 2006 Sep;8(9):1488-503. doi: 10.1111/j.1462-5822.2006.00728.x. Cell Microbiol. 2006. PMID: 16922867
-
Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly.FEBS J. 2010 Jun;277(11):2390-402. doi: 10.1111/j.1742-4658.2010.07653.x. Epub 2010 Apr 27. FEBS J. 2010. PMID: 20477869 Review.
-
Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7.Curr Opin Microbiol. 2003 Feb;6(1):82-90. doi: 10.1016/s1369-5274(03)00005-5. Curr Opin Microbiol. 2003. PMID: 12615225 Review.
Cited by
-
Infection strategies of enteric pathogenic Escherichia coli.Gut Microbes. 2012 Mar-Apr;3(2):71-87. doi: 10.4161/gmic.19182. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22555463 Free PMC article. Review.
-
The ability of an attaching and effacing pathogen to trigger localized actin assembly contributes to virulence by promoting mucosal attachment.Cell Microbiol. 2014 Sep;16(9):1405-24. doi: 10.1111/cmi.12302. Epub 2014 Jun 2. Cell Microbiol. 2014. PMID: 24780054 Free PMC article.
-
Why put yourself on a pedestal? The pathogenic role of the A/E pedestal.Infect Immun. 2024 Sep 10;92(9):e0048923. doi: 10.1128/iai.00489-23. Epub 2024 Apr 9. Infect Immun. 2024. PMID: 38591884 Free PMC article. Review.
-
Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation.PLoS Pathog. 2010 Aug 19;6(8):e1001056. doi: 10.1371/journal.ppat.1001056. PLoS Pathog. 2010. PMID: 20808845 Free PMC article.
-
Cortactin recruitment by enterohemorrhagic Escherichia coli O157:H7 during infection in vitro and ex vivo.Infect Immun. 2008 Oct;76(10):4669-76. doi: 10.1128/IAI.00140-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678675 Free PMC article.
References
-
- Berceli SA, Borovetz HS, Sheppeck RA, Moosa HH, Warty VS, Armany MA, Herman IM. Mechanisms of vein graft atherosclerosis: LDL metabolism and endothelial actin reorganization. J Vasc Surg. 1991;13:336–347. - PubMed
-
- Brady MJ, Campellone KG, Ghildiyal M, Leong JM. Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell Microbiol 2007 - PubMed
-
- Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. - PubMed
-
- Campellone KG, Giese A, Tipper DJ, Leong JM. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol Microbiol. 2002;43:1227–1241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

