Expression of AID transgene is regulated in activated B cells but not in resting B cells and kidney
- PMID: 18067961
- PMCID: PMC2376253
- DOI: 10.1016/j.molimm.2007.10.041
Expression of AID transgene is regulated in activated B cells but not in resting B cells and kidney
Abstract
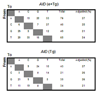
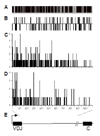
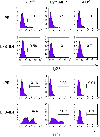
Activation-induced DNA cytidine deaminase (AID) is required for somatic hypermutation (SHM) and efficient class switch recombination (CSR) of immunoglobulin (Ig) genes. We created AID-transgenic mice that express AID ubiquitously under the control of a beta-actin promoter. When crossed with AID-/- mice, the AID-transgenic,AID-/- mice carried out SHM and CSR, showing that the AID transgenes were functional. However, the frequencies of SHM in V- and switch-regions, and CSR were reduced compared to those in a wild type AID background. Several criteria suggested that the inefficiency of SHM was due to reduced AID activity, rather than lack of recruiting error-prone DNA repair. High levels of AID mRNA were produced in resting B cells and kidney, cells that do not express AID in wild type mice. Compared with these cells, activated B cells expressed about an order of magnitude less AID mRNA suggesting that there may be a post-transcriptional mechanism that regulates AID mRNA levels in professional AID producers but not other cells. The AID protein expressed in resting B cells and kidney was phosphorylated at serine-38. Despite this modification, known to enhance AID activity, resting B cells did not undergo SHM. Apparently, the large amounts of AID in resting B cells are not targeted to Ig genes in vivo, in contrast to findings in vitro.
Figures




References
-
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. - PubMed
-
- Basu U, Chaudhuri J, Alpert C, Dutt S, Ranganath S, Li G, Schrum J, Manis J, Alt F. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 2005;438:508–511. - PubMed
-
- Camacho SA, Kosco-Vilbois MH, Berek C. The dynamic structure of the germinal center. Immunol Today. 1998;19:511–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

