Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor
- PMID: 18068633
- PMCID: PMC2931420
- DOI: 10.1016/j.ccr.2007.11.002
Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor
Abstract
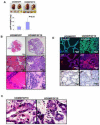
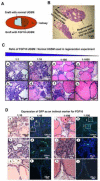
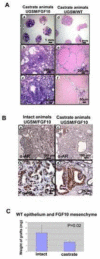
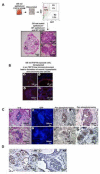
Enhanced mesenchymal expression of FGF10 led to the formation of multifocal PIN or prostate cancer. Inhibition of epithelial FGFR1 signaling using DN FGFR1 led to reversal of the cancer phenotype. A subset of the FGF10-induced carcinoma was serially transplantable. Paracrine FGF10 led to an increase in epithelial androgen receptor and synergized with cell-autonomous activated AKT. Our observations indicate that stromal FGF10 expression may facilitate the multifocal histology observed in prostate adenocarcinoma and suggest the FGF10/FGFR1 axis as a potential therapeutic target in treating hormone-sensitive or refractory prostate cancer. We also show that transient exposure to a paracrine growth factor may be sufficient for the initiation of oncogenic transformation.
Figures



References
-
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004a;303:848–851. - PubMed
-
- Bostwick DG, Foster CS. Predictive factors in prostate cancer: current concepts from the 1999 College of American Pathologists Conference on Solid Tumor Prognostic Factors and the 1999 World Health Organization Second International Consultation on Prostate Cancer. Semin Urol Oncol. 1999;17:222–272. - PubMed
-
- Boxer RB, Jang JW, Sintasath L, Chodosh LA. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–586. - PubMed
-
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 HD000849/HD/NICHD NIH HHS/United States
- R01 CA107300/CA/NCI NIH HHS/United States
- R01 CA090571/CA/NCI NIH HHS/United States
- K12-CA076905-09/CA/NCI NIH HHS/United States
- R01CA90571/CA/NCI NIH HHS/United States
- K12 CA076905/CA/NCI NIH HHS/United States
- R01CA107300/CA/NCI NIH HHS/United States
- 5K12HD00849-18/HD/NICHD NIH HHS/United States
- R01 CA107166/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K99 CA125937/CA/NCI NIH HHS/United States
- 1K99CA125937/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

