Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat
- PMID: 18068901
- PMCID: PMC2543943
- DOI: 10.1016/j.pain.2007.10.017
Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat
Abstract
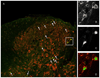
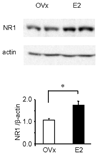
Pain symptoms in several chronic pain disorders in women, including irritable bowel syndrome, fluctuate with the menstrual cycle suggesting a gonadal hormone component. In female rats, estrogens modulate visceral sensitivity although the underlying mechanism(s) are unknown. In the present study the effects of 17-beta estradiol on N-methyl-D-aspartate (NMDA) receptor signaling of colorectal nociceptive processing in the spinal cord were examined. Estrogen receptor alpha and the NR1 subunit of the NMDA receptor are co-expressed in dorsal horn neurons, supporting a direct action of estradiol on NMDA receptors. Intrathecal administration of the NMDA receptor antagonist D(-)-2-amino-5-phosphonopentanoic acid (APV) dose-dependently attenuated the visceromotor response with greater potency in ovariectomized (OVx) rats compared to OVx with estradiol replacement (E2) rats. Estradiol significantly increased protein expression of NR1 in the lumbosacral spinal cord compared to OVx rats. Colorectal distention significantly increased phosphorylation of NR1ser-897, a PKA phosphorylation site on the NR1 subunit in E2, but not OVx rats. Intrathecal administration of a PKA inhibitor significantly attenuated the visceromotor response, decreased NR1 phosphorylation and increased the potency of APV to attenuate the visceromotor response compared to vehicle-treated E2 rats. These data suggest that estradiol increases spinal processing of visceral nociception by increasing NMDA receptor NR1 subunit expression and increasing site-specific receptor phosphorylation on the NR1 subunit contributing to an increase in NMDA receptor activity.
Figures




References
-
- Adams MM, Oung T, Morrison JH, Gore AC. Length of postovariectomy interval and age, but not estrogen replacement, regulate N-methyl-D-aspartate receptor mRNA levels in the hippocampus of female rats. Exp.Neurol. 2001;170:345–356. - PubMed
-
- Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci.Lett. 1995;196:25–28. - PubMed
-
- Berkley KJ. Sex differences in pain. Behav.Brain Sci. 1997;20:371–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

