Eukaryotic initiation factor 4E
- PMID: 18069043
- PMCID: PMC3061223
- DOI: 10.1016/j.biocel.2007.10.023
Eukaryotic initiation factor 4E
Abstract
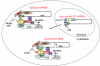
Eukaryotic translation initiation factor 4E (eIF4E) is perhaps best known for its function in the initiation of protein synthesis on capped mRNAs in the cytoplasm. However, recent studies have highlighted that eIF4E has many additional functions, which include the nuclear export of specific mRNAs as well as roles in ageing and the translation of some uncapped viral RNAs. This review aims to update the reader on recent developments, including the potential of eIF4E as a therapeutic target.
Figures

References
-
- Belsham GJ, Jackson RJ. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; 2000. Translation initiation on picornavirus RNA; pp. 869–900.
-
- Chaudhry Y, Nayak A, Bordeleau ME, Tanaka J, Pelletier J, Belsham GJ, Roberts LO, Goodfellow IG. Caliciviruses differ in their functional requirements for eIF4F components. J. Biol. Chem. 2006;281:25315–25325. - PubMed
-
- Culjkovic B, Topisirovic I, Borden KLB. Controlling gene expression through RNA regulons. Cell Cycle. 2007;6:65–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

