Respiratory plasticity after perinatal hyperoxia is not prevented by antioxidant supplementation
- PMID: 18069076
- PMCID: PMC2431464
- DOI: 10.1016/j.resp.2007.10.013
Respiratory plasticity after perinatal hyperoxia is not prevented by antioxidant supplementation
Abstract
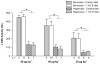
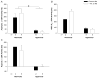
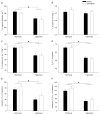
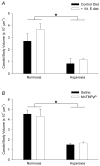
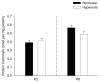
Perinatal hyperoxia attenuates the hypoxic ventilatory response in rats by altering development of the carotid body and its chemoafferent neurons. In this study, we tested the hypothesis that hyperoxia elicits this plasticity through the increased production of reactive oxygen species (ROS). Rats were born and raised in 60% O(2) for the first two postnatal weeks while treated with one of two antioxidants: vitamin E (via milk from mothers whose diet was enriched with 1000 IU vitamin E kg(-1)) or a superoxide dismutase mimetic, manganese(III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP; via daily intraperitoneal injection of 5-10 mg kg(-1)); rats were subsequently raised in room air until studied as adults. Peripheral chemoreflexes, assessed by carotid sinus nerve responses to cyanide, asphyxia, anoxia and isocapnic hypoxia (vitamin E experiments) or by hypoxic ventilatory responses (MnTMPyP experiments), were reduced after perinatal hyperoxia compared to those of normoxia-reared controls (all P<0.01); antioxidant treatment had no effect on these responses. Similarly, the carotid bodies of hyperoxia-reared rats were only one-third the volume of carotid bodies from normoxia-reared controls (P <0.001), regardless of antioxidant treatment. Protein carbonyl concentrations in the blood plasma, measured as an indicator of oxidative stress, were not increased in neonatal rats (2 and 8 days of age) exposed to 60% O(2) from birth. Collectively, these data do not support the hypothesis that perinatal hyperoxia impairs peripheral chemoreceptor development through ROS-mediated oxygen toxicity.
Figures
References
-
- Bavis RW. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir Physiol Neurobiol. 2005;149:287–299. - PubMed
-
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol. 2002;92:1013–1018. - PubMed
-
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol. 2003;95:1550–1559. - PubMed
-
- Bavis RW, Russell KER, Simons JC, Otis JP. Hypoxic ventilatory responses in rats after hypercapnic hyperoxia and intermittent hyperoxia. Respir Physiol Neurobiol. 2007;155:193–202. - PubMed
-
- Bavis RW, Powell FL, Bradford A, Hsia CCW, Peltonen JE, Soliz J, Zeis B, Fergusson EK, Fu Z, Gassmann M, Kim CB, Maurer J, McGuire M, Miller BM, O’Halloran KD, Paul RJ, Reid SG, Rusko HK, Tikkanen HO, Wilkinson KA. Respiratory plasticity in response to changes in oxygen supply and demand. Integr Comp Biol. In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

