The modular organization of domain structures: insights into protein-protein binding
- PMID: 18069884
- PMCID: PMC2134966
- DOI: 10.1371/journal.pcbi.0030239
The modular organization of domain structures: insights into protein-protein binding
Abstract
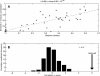
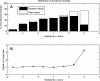
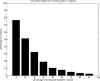
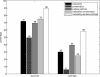
Domains are the building blocks of proteins and play a crucial role in protein-protein interactions. Here, we propose a new approach for the analysis and prediction of domain-domain interfaces. Our method, which relies on the representation of domains as residue-interacting networks, finds an optimal decomposition of domain structures into modules. The resulting modules comprise highly cooperative residues, which exhibit few connections with other modules. We found that non-overlapping binding sites in a domain, involved in different domain-domain interactions, are generally contained in different modules. This observation indicates that our modular decomposition is able to separate protein domains into regions with specialized functions. Our results show that modules with high modularity values identify binding site regions, demonstrating the predictive character of modularity. Furthermore, the combination of modularity with other characteristics, such as sequence conservation or surface patches, was found to improve our predictions. In an attempt to give a physical interpretation to the modular architecture of domains, we analyzed in detail six examples of protein domains with available experimental binding data. The modular configuration of the TEM1-beta-lactamase binding site illustrates the energetic independence of hotspots located in different modules and the cooperativity of those sited within the same modules. The energetic and structural cooperativity between intramodular residues is also clearly shown in the example of the chymotrypsin inhibitor, where non-binding site residues have a synergistic effect on binding. Interestingly, the binding site of the T cell receptor beta chain variable domain 2.1 is contained in one module, which includes structurally distant hot regions displaying positive cooperativity. These findings support the idea that modules possess certain functional and energetic independence. A modular organization of binding sites confers robustness and flexibility to the performance of the functional activity, and facilitates the evolution of protein interactions.
Conflict of interest statement
Figures


References
-
- Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–452. - PubMed
-
- Aasland R, Abrams C, Ampe C, Ball LJ, Bedford MT, et al. Normalization of nomenclature for peptide motifs as ligands of modular protein domains. FEBS Lett. 2002;513:141–144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

