A RNA interference screen identifies the protein phosphatase 2A subunit PR55gamma as a stress-sensitive inhibitor of c-SRC
- PMID: 18069897
- PMCID: PMC2134945
- DOI: 10.1371/journal.pgen.0030218
A RNA interference screen identifies the protein phosphatase 2A subunit PR55gamma as a stress-sensitive inhibitor of c-SRC
Abstract
Protein Phosphatase type 2A (PP2A) represents a family of holoenzyme complexes with diverse biological activities. Specific holoenzyme complexes are thought to be deregulated during oncogenic transformation and oncogene-induced signaling. Since most studies on the role of this phosphatase family have relied on the use of generic PP2A inhibitors, the contribution of individual PP2A holoenzyme complexes in PP2A-controlled signaling pathways is largely unclear. To gain insight into this, we have constructed a set of shRNA vectors targeting the individual PP2A regulatory subunits for suppression by RNA interference. Here, we identify PR55gamma and PR55delta as inhibitors of c-Jun NH(2)-terminal kinase (JNK) activation by UV irradiation. We show that PR55gamma binds c-SRC and modulates the phosphorylation of serine 12 of c-SRC, a residue we demonstrate to be required for JNK activation by c-SRC. We also find that the physical interaction between PR55gamma and c-SRC is sensitive to UV irradiation. Our data reveal a novel mechanism of c-SRC regulation whereby in response to stress c-SRC activity is regulated, at least in part, through loss of the interaction with its inhibitor, PR55gamma.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures





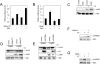


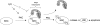
References
-
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. - PubMed
-
- Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. - PubMed
-
- Zheng XM, Wang Y, Pallen CJ. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992;359:336–339. - PubMed
-
- Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. 2000;275:41439–41446. - PubMed
-
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

