Early treatment suppresses the development of spike-wave epilepsy in a rat model
- PMID: 18070091
- PMCID: PMC3143182
- DOI: 10.1111/j.1528-1167.2007.01458.x
Early treatment suppresses the development of spike-wave epilepsy in a rat model
Abstract
Purpose: Current treatments for epilepsy may control seizures, but have no known effects on the underlying disease. We sought to determine whether early treatment in a model of genetic epilepsy would reduce the severity of the epilepsy phenotype in adulthood.
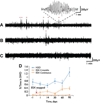
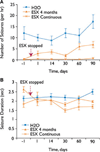
Methods: We used Wistar albino Glaxo rats of Rijswijk (WAG/Rij) rats, an established model of human absence epilepsy. Oral ethosuximide was given from age p21 to 5 months, covering the usual period in which seizures develop in this model (age approximately 3 months). Two experiments were performed: (1) cortical expression of ion channels Nav1.1, Nav1.6, and HCN1 (previously shown to be dysregulated in WAG/Rij) measured by immunocytochemistry in adult treated rats; and (2) electroencephalogram (EEG) recordings to measure seizure severity at serial time points after stopping the treatment.
Results: Early treatment with ethosuximide blocked changes in the expression of ion channels Nav1.1, Nav1.6, and HCN1 normally associated with epilepsy in this model. In addition, the treatment led to a persistent suppression of seizures, even after therapy was discontinued. Thus, animals treated with ethosuximide from age p21 to 5 months still had a marked suppression of seizures at age 8 months.
Discussion: These findings suggest that early treatment during development may provide a new strategy for preventing epilepsy in susceptible individuals. If confirmed with other drugs and epilepsy paradigms, the availability of a model in which epileptogenesis can be controlled has important implications both for future basic studies, and human therapeutic trials.
Figures



References
-
- Avoli M, Rogawski MA, Avanzini G. Generalized epileptic disorders: an update. Epilepsia. 2001;42:445–457. - PubMed
-
- Bartolomei F, Gastaldi M, Massacrier A, Planells R, Nicolas S, Cau P. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J Neurocytol. 1997;26:667–678. - PubMed
-
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. - PubMed
-
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–6836. - PMC - PubMed
-
- Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

