The microtubule-associated protein tau is phosphorylated by Syk
- PMID: 18070606
- PMCID: PMC2258316
- DOI: 10.1016/j.bbamcr.2007.11.005
The microtubule-associated protein tau is phosphorylated by Syk
Abstract
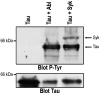
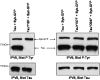
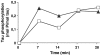
Aberrant phosphorylation of tau protein on serine and threonine residues has been shown to be critical in neurodegenerative disorders called tauopathies. An increasing amount of data suggest that tyrosine phosphorylation of tau might play an equally important role in pathology, with at least three putative tyrosine kinases of tau identified to date. It was recently shown that the tyrosine kinase Syk could efficiently phosphorylate alpha-synuclein, the aggregated protein found in Parkinson's disease and other synucleinopathies. We report herein that Syk is also a tau kinase, phosphorylating tau in vitro and in CHO cells when both proteins are expressed exogenously. In CHO cells, we have also demonstrated by co-immunoprecipitation that Syk binds to tau. Finally, by site-directed mutagenesis substituting the tyrosine residues of tau with phenylalanine, we established that tyrosine 18 was the primary residue in tau phosphorylated by Syk. The identification of Syk as a common tyrosine kinase of both tau and alpha-synuclein may be of potential significance in neurodegenerative disorders and also in neuronal physiology. These results bring another clue to the intriguing overlaps between tauopathies and synucleinopathies and provide new insights into the role of Syk in neuronal physiology.
Figures

References
-
- Norris E.H., Giasson B.I., Lee V.M. Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr. Top. Dev. Biol. 2004;60:17–54. - PubMed
-
- Sergeant N., Delacourte A., Buee L. Tau protein as a differential biomarker of tauopathies. Biochim. Biophys. Acta. 2005;1739:179–197. - PubMed
-
- Anderton B.H., Betts J., Blackstock W.P., Brion J.P., Chapman S., Connell J., Dayanandan R., Gallo J.M., Gibb G., Hanger D.P., Hutton M., Kardalinou E., Leroy K., Lovestone S., Mack T., Reynolds C.H., Van Slegtenhorst M. Sites of phosphorylation in tau and factors affecting their regulation. Biochem. Soc. Symp. 2001:73–80. - PubMed
-
- Hanger D., Byers H.L., Wray S., Leung K.Y., Saxton M.J., Seereeram A., Reynolds C.H., Ward M.A., Anderton B. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem. 2007;282:23645–23654. - PubMed
-
- Lovestone S., Reynolds C.H. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

