Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart
- PMID: 18070934
- PMCID: PMC2150988
- DOI: 10.1084/jem.20070166
Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart
Abstract
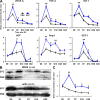
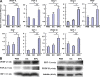
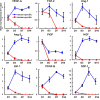
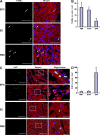
Noncellular differentiation effects have emerged as important mechanisms mediating therapeutic effects of stem or progenitor cell transplantation. Here, we investigated the expression patterns and sources of humoral factors and their regional and systemic biological effects after bone marrow (BM)-derived endothelial progenitor cell (EPC) transplantation into ischemic myocardium. Although most of the transplanted EPCs disappeared within a week, up-regulation of multiple humoral factors was sustained for longer than two weeks, which correlated well with the recovery of cardiac function. To determine the source of the humoral factors, we injected human EPCs into immunodeficient mice. Whereas the expression of human EPC (donor)-derived cytokines rapidly decreased to a nondetectable level within a week, up-regulation of mouse (recipient)-derived cytokines, including factors that could mobilize BM cells, was sustained. Histologically, we observed higher capillary density, a higher proliferation of myocardial cells, a lower cardiomyocyte apoptosis, and reduced infarct size. Furthermore, after EPC transplantation, BM-derived stem or progenitor cells were increased in the peripheral circulation and incorporated into the site of neovascularization and myocardial repair. These data indicate that myocardial EPC transplantation induces humoral effects, which are sustained by host tissues and play a crucial role in repairing myocardial injury.
Figures



References
-
- WHO. World Health Report. www.who.int/whr/
-
- Orlic, D., J. Kajstura, S. Chimenti, I. Jakoniuk, S.M. Anderson, B. Li, J. Pickel, R. McKay, B. Nadal-Ginard, D.M. Bodine, et al. 2001. Bone marrow cells regenerate infarcted myocardium. Nature. 410:701–705. - PubMed
-
- Kocher, A.A., M.D. Schuster, M.J. Szabolcs, S. Takuma, D. Burkhoff, J. Wang, S. Homma, N.M. Edwards, and S. Itescu. 2001. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med. 7:430–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

