A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids
- PMID: 18071293
- PMCID: PMC2275445
- DOI: 10.1038/sj.bjp.0707627
A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids
Abstract
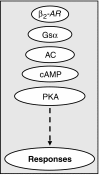
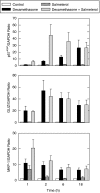
There is unequivocal evidence that the combination of an inhaled corticosteroid (ICS) -- i.e. glucocorticoid -- and an inhaled long-acting beta(2)-adrenoceptor agonist (LABA) is superior to each component administered as a monotherapy alone in the clinical management of asthma. Moreover, Calverley and colleagues (Lancet 2003, 361: 449-456; N Engl J Med 2007, 356: 775-789) reporting for the 'TRial of Inhaled STeroids ANd long-acting beta(2)-agonists (TRISTAN)' and 'TOwards a Revolution in COPD Health (TORCH)' international study groups also demonstrated the superior efficacy of LABA/ICS combination therapies over ICS alone in the clinical management of chronic obstructive pulmonary disease. This finding has been independently confirmed indicating that the therapeutic benefit of LABA/ICS combination therapies is not restricted to asthma and may be extended to other chronic inflammatory diseases of the airways. Despite the unquestionable benefit of LABA/ICS combination therapies, there is a vast gap in our understanding of how these two drugs given together deliver superior clinical efficacy. In this article, we review the history of LABA/ICS combination therapies and critically evaluate how these two classes of drugs might interact at the biochemical level to suppress pro-inflammatory responses. Understanding the molecular basis of this fundamental clinical observation is a Holy Grail of current respiratory diseases research as it could permit the rational exploitation of this effect with the development of new 'optimized' LABA/ICS combination therapies.
Figures

Comment in
-
Combining inhaled glucocorticoids and long acting beta(2)-adrenoceptor agonists in asthma and COPD.Br J Pharmacol. 2008 Mar;153(6):1085-6. doi: 10.1038/bjp.2008.4. Epub 2008 Jan 28. Br J Pharmacol. 2008. PMID: 18223662 Free PMC article.
References
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–555. - PubMed
-
- Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol. 2004;27:35–44. - PubMed
-
- Barnes NC, Qiu YS, Pavord ID, Parker D, Davis PA, Zhu J, et al. Anti-inflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;173:736–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

