Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo
- PMID: 18071337
- PMCID: PMC2930752
- DOI: 10.1038/sj.mt.6300343
Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo
Abstract
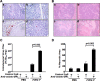
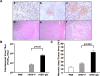
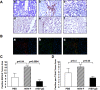
Oncolytic virotherapy is a promising strategy for treatment of malignancy, although its effectiveness is hampered by host antiviral inflammatory responses. The efficacy of treatment of oncolytic vesicular stomatitis virus (VSV) in rats bearing multifocal hepatocellular carcinoma (HCC) can be substantially elevated by antibody-mediated depletion of natural killer (NK) cells. In order to test the hypothesis that the oncotyic potency of VSV can be exponentially elevated by evasion of inflammatory responses in vivo, we constructed a recombinant VSV vector expressing equine herpes virus-1 glycoprotein G, which is a broad-spectrum viral chemokine binding protein (rVSV-gG). Infusion of rVSV-gG via the hepatic artery into immune-competent rats bearing syngeneic and multifocal HCC in their livers, resulted in a reduction of NK and NKT cells in the tumors and a 1-log enhancement in intratumoral virus titer in comparison with a reference rVSV vector. The treatment led to increased tumor necrosis and substantially prolonged animal survival without toxicities. These results indicate that rVSV-gG has the potential to be developed as an effective and safe oncolytic agent to treat patients with advanced HCC. Furthermore, the novel concept that oncolytic potency can be substantially enhanced by vector-mediated suppression of host antiviral inflammatory responses could have general applicability in the field of oncolytic virotherapy for cancer.
Figures
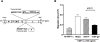
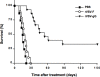

References
-
- Murray CJ, L AD. Evidence-based health policy - lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. - PubMed
-
- El-Serag HB, M AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. - PubMed
-
- Dyer Z, Peltekian K, van Zanten SV. The changing epidemiology of hepatocellular carcinoma in Canada. Aliment Pharmacol Ther. 2005;22:17–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

