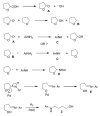
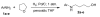A new palladium-mediated approach to 4-N-arylamino-1-butanols from peroxidic tetrahydrofuran and primary aromatic amines
- PMID: 18071580
- PMCID: PMC2128685
- DOI: 10.1016/j.tetlet.2006.12.136
A new palladium-mediated approach to 4-N-arylamino-1-butanols from peroxidic tetrahydrofuran and primary aromatic amines
Abstract
Reaction of primary aromatic amines with peroxidic tetrahydrofuran (THF) in the presence of hydrogen and 10% palladium on carbon catalyst results in THF ring opening to give 4-N-arylamino-1-butanols in good yield. The reaction mechanism is believed to involve a free-radical sequence resulting in an imino alcohol subsequently reduced to product.
Figures
References
-
- Wichterle O. Collect Czech Chem Commun. 1949;14:209–18.
-
- Lunsford CD, Murphy RS, Rose EK. J Org Chem. 1957;22:1225–1228.
-
- Flanikin JM, Collins JC, Lanz M, Singaram B. Organic Letters. 1999;1:799–801. - PubMed
-
- Winberg HE. US Patent. 2,628,978. 1953.
-
- McCalmont WF, Patterson JR, Lindenmuth MA, Heady TN, Haverstick DM, Gray LS, Macdonald TL. Bioorg Med Chem. 2005;13:3821–39. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources