Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases
- PMID: 18071913
- PMCID: PMC2730112
- DOI: 10.1007/s10585-007-9133-3
Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases
Abstract
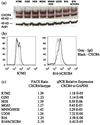
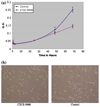
Metastasis continues to be the leading cause of mortality for patients with cancer. High expression of the chemokine receptor CXCR4 correlates with poor prognosis in many cancers, including osteosarcoma and melanoma. CXCL12, the ligand for CXCR4, is expressed at high levels in the lung and lymph node, which are the primary sites to which these tumors metastasize respectively. These findings suggest that therapy aimed at disruption of this specific receptor/ligand complex may lead to a decrease in metastases. CTCE-9908, a small peptide CXCR4 antagonist was utilized in two murine metastasis models to test this hypothesis. Treatment of osteosarcoma cells in vitro with CTCE-9908 led to the following changes: decreased adhesion, decreased migration, decreased invasion, and decreased growth rate. Following tail vein injection of osteosarcoma cells, mice that were treated with CTCE-9908 had a 50% reduction in the number of gross metastatic lung nodules and a marked decrease in micro-metastatic disease. Similar findings were observed following injection of melanoma cells and treatment with CTCE-9908. However, these results could only be consistently reproduced when the cells were pre-treated with the inhibitor. A novel ex vivo luciferase assay showed decreased numbers of cells in the lung immediately after injection into mice, when treated with CTCE-9908, suggesting the importance of interactions between the receptor and the ligand. Our findings show that inhibition of the CXCR4/CXCL12 pathway decreases metastatic disease in two murine tumor models and expands on previous reports to describe potential mechanisms of action.
Figures






References
-
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. - PubMed
-
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. - PubMed
-
- Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. - PubMed
-
- Feng Y, Broder CC, Kennedy PE, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. - PubMed
-
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

