The NMDA receptor NR1 C1 region bound to calmodulin: structural insights into functional differences between homologous domains
- PMID: 18073110
- PMCID: PMC2246159
- DOI: 10.1016/j.str.2007.10.012
The NMDA receptor NR1 C1 region bound to calmodulin: structural insights into functional differences between homologous domains
Abstract
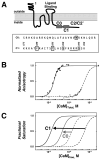
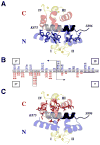
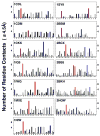
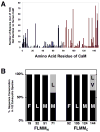
Calmodulin (CaM) regulates tetrameric N-methyl-D-aspartate receptors (NMDARs) by binding tightly to the C0 and C1 regions of its NR1 subunit. A crystal structure (2HQW; 1.96 A) of calcium-saturated CaM bound to NR1C1 (peptide spanning 875-898) showed that NR1 S890, whose phosphorylation regulates membrane localization, was solvent protected, whereas the endoplasmic reticulum retention motif was solvent exposed. NR1 F880 filled the CaM C-domain pocket, whereas T886 was closest to the N-domain pocket. This 1-7 pattern was most similar to that in the CaM-MARCKS complex. Comparison of CaM-ligand wrap-around conformations identified a core tetrad of CaM C-domain residues (FLMM(C)) that contacted all ligands consistently. An identical tetrad of N-domain residues (FLMM(N)) made variable sets of contacts with ligands. This CaM-NR1C1 structure provides a foundation for designing mutants to test the role of CaM in NR1 trafficking as well as insights into how the homologous CaM domains have different roles in molecular recognition.
Figures





References
-
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71:713–716. - PubMed
-
- Akyol Z, Bartos JA, Merrill MA, Faga LA, Jaren OR, Shea MA, Hell JW. Apo–Calmodulin Binds with its COOH-terminal Domain to the N-methyl-D-aspartate Receptor NR1 C0 Region. J Biol Chem. 2004;279:2166–2175. - PubMed
-
- Bhattacharya S, Bunick CG, Chazin WJ. Target selectivity in EF-hand calcium binding proteins. Biochim Biophys Acta. 2004;1742:69–79. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

