Motor coordination deficits in mice lacking RGS9
- PMID: 18073128
- PMCID: PMC2241663
- DOI: 10.1016/j.brainres.2007.11.017
Motor coordination deficits in mice lacking RGS9
Abstract
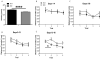
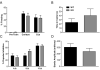
RGS9-2 is a striatum-enriched protein that negatively modulates dopamine and opioid receptor signaling. We examined the role of RGS9-2 in modulating complex behavior. Genetic deletion of RGS9-2 does not lead to global impairments, but results in selective abnormalities in certain behavioral domains. RGS9 knockout (KO) mice have decreased motor coordination on the accelerating rotarod and deficits in working memory as measured in the delayed-match-to-place version of the water maze. In contrast, RGS9 KO mice exhibit normal locomotor activity, anxiety-like behavior, cue and contextual fear conditioning, startle threshold, and pre-pulse inhibition. These studies are the first to describe a role for RGS9-2 in motor coordination and working memory and implicate RGS9-2 as a potential therapeutic target for motor and cognitive dysfunction.
Figures

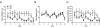
References
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–30. - PubMed
-
- Battig K, Rosvold HE, Mishkin M. Comparison of the effects of frontal and caudate lesions on delayed response and alternation in monkeys. J Comp Physiol Psychol. 1960;53:400–4. - PubMed
-
- Berman DM, Kozasa T, Gilman AG. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem. 1996;271:27209–12. - PubMed
-
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. J Biol Chem. 1998;273:1269–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

