Protein kinase A-independent inhibition of proliferation and induction of apoptosis in human thyroid cancer cells by 8-Cl-adenosine
- PMID: 18073299
- PMCID: PMC2266951
- DOI: 10.1210/jc.2007-2331
Protein kinase A-independent inhibition of proliferation and induction of apoptosis in human thyroid cancer cells by 8-Cl-adenosine
Abstract
Purpose: Protein kinase A (PKA) affects cell proliferation in many cell types and is a potential target for cancer treatment. PKA activity is stimulated by cAMP and cAMP analogs. One such substance, 8-Cl-cAMP, and its metabolite 8-Cl-adenosine (8-Cl-ADO) are known inhibitors of cancer cell proliferation; however, their mechanism of action is controversial. We have investigated the antiproliferative effects of 8-Cl-cAMP and 8-CL-ADO on human thyroid cancer cells and determined PKA's involvement.
Experimental design: We employed proliferation and apoptosis assays and PKA activity and cell cycle analysis to understand the effect of 8-Cl-ADO and 8-Cl-cAMP on human thyroid cancer and HeLa cell lines.
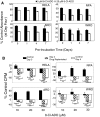
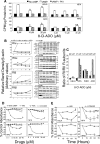
Results: 8-Cl-ADO inhibited proliferation of all cells, an effect that lasted for at least 4 d. Proliferation was also inhibited by 8-Cl-cAMP, but this inhibition was reduced by 3-isobutyl-1-methylxanthine; both drugs stimulated apoptosis, and 3-isobutyl-1-methylxanthine drastically reduced 8-Cl-cAMP-induced cell death. 8-Cl-ADO induced cell accumulation in G1/S or G2/M cell cycle phases and differentially altered PKA activity and subunit levels. PKA stimulation or inhibition and adenosine receptor agonists or antagonists did not significantly affect proliferation.
Conclusions: 8-Cl-ADO and 8-Cl-cAMP inhibit proliferation, induce cell cycle phase accumulation, and stimulate apoptosis in thyroid cancer cells. The effect of 8-Cl-cAMP is likely due to its metabolite 8-Cl-ADO, and PKA does not appear to have direct involvement in the inhibition of proliferation by 8-Cl-ADO. 8-Cl-ADO may be a useful therapeutic agent to be explored in aggressive thyroid cancer.
Figures




References
-
- Sakamoto A 2004 Definition of poorly differentiated carcinoma of the thyroid: the Japanese experience. Endocr Pathol 15:307–311 - PubMed
-
- Nikiforov YE 2004 Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol 15:319–327 - PubMed
-
- Delellis RA, Lloyd RV, Hertz PU, Eng C 2004 Tumors of the thyroid and parathyroid. In: Delellis RA, Lloyd RV, Hertz PU, Eng C, eds. WHO classification of tumors: pathology and genetics. Lyon, France: IARC Press; 51–80
-
- Liska J, Altanerova V, Galbavy S, Stvrtina S, Brtko J 2005 Thyroid tumors: histological classification and genetic factors involved in the development of thyroid cancer. Endocr Res 39:73–83 - PubMed
-
- Ain KB 1998 Anaplastic thyroid carcinoma: behavior, biology and therapeutic approaches. Thyroid 8:715–726 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

