The reacquisition of biotin prototrophy in Saccharomyces cerevisiae involved horizontal gene transfer, gene duplication and gene clustering
- PMID: 18073433
- PMCID: PMC2219469
- DOI: 10.1534/genetics.107.074963
The reacquisition of biotin prototrophy in Saccharomyces cerevisiae involved horizontal gene transfer, gene duplication and gene clustering
Abstract
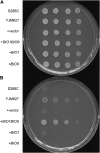
The synthesis of biotin, a vitamin required for many carboxylation reactions, is a variable trait in Saccharomyces cerevisiae. Many S. cerevisiae strains, including common laboratory strains, contain only a partial biotin synthesis pathway. We here report the identification of the first step necessary for the biotin synthesis pathway in S. cerevisiae. The biotin auxotroph strain S288c was able to grow on media lacking biotin when BIO1 and the known biotin synthesis gene BIO6 were introduced together on a plasmid vector. BIO1 is a paralog of YJR154W, a gene of unknown function and adjacent to BIO6. The nature of BIO1 illuminates the remarkable evolutionary history of the biotin biosynthesis pathway in S. cerevisiae. This pathway appears to have been lost in an ancestor of S. cerevisiae and subsequently rebuilt by a combination of horizontal gene transfer and gene duplication followed by neofunctionalization. Unusually, for S. cerevisiae, most of the genes required for biotin synthesis in S. cerevisiae are grouped in two subtelomeric gene clusters. The BIO1-BIO6 functional cluster is an example of a cluster of genes of "dispensable function," one of the few categories of genes in S. cerevisiae that are positionally clustered.
Figures










References
-
- Abe, Y., T. Suzuki, C. Ono, K. Iwamoto, M. Hosobuchi et al., 2002. Molecular cloning and characterization of an ML-236B (compactin) biosynthetic gene cluster in Penicillium citrinum. Mol. Genet Genomics 267: 636–646. - PubMed
-
- Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li et al., 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288c: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

