Modulation of bacterial Type III secretion system by a spermidine transporter dependent signaling pathway
- PMID: 18074016
- PMCID: PMC2110884
- DOI: 10.1371/journal.pone.0001291
Modulation of bacterial Type III secretion system by a spermidine transporter dependent signaling pathway
Abstract
Background: Many gram-negative bacterial pathogens employ Type III secretion systems (T3SS) to inject effector proteins into host cells in infectious processes.
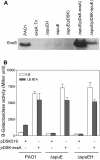
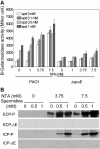
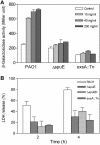
Methodology/principal findings: By screening a transposon mutant library of P. aeruginosa, we found that mutation of spuDEFGH, which encode a major spermidine uptake system, abolished the expression of the exsCEBA operon that codes for key T3SS regulators under inducing conditions (low calcium). Whole genome microarray analysis revealed that inactivation of the spermidine uptake system significantly decreased the transcriptional expression of most, if not all, T3SS genes. Consistently, the spermidine uptake mutants showed decreased expression of the T3SS genes in responding to host cell extract and attenuated cytotoxicity. Furthermore, exogenous addition of spermidine to the wild type strain PAO1 enhanced the expression of exsCEBA and also the effector protein genes.
Conclusion/significance: Cumulatively, these data have depicted a novel spermidine transporter-dependent signaling pathway, which appears to play an essential role in modulation of T3SS expression in P. aeruginosa.
Conflict of interest statement
Figures


Similar articles
-
Spermidine Is an Intercellular Signal Modulating T3SS Expression in Pseudomonas aeruginosa.Microbiol Spectr. 2022 Jun 29;10(3):e0064422. doi: 10.1128/spectrum.00644-22. Epub 2022 Apr 18. Microbiol Spectr. 2022. PMID: 35435755 Free PMC article.
-
Pseudomonas aeruginosa Magnesium Transporter MgtE Inhibits Type III Secretion System Gene Expression by Stimulating rsmYZ Transcription.J Bacteriol. 2017 Oct 31;199(23):e00268-17. doi: 10.1128/JB.00268-17. Print 2017 Dec 1. J Bacteriol. 2017. PMID: 28847924 Free PMC article.
-
tRNA modification enzyme MiaB connects environmental cues to activation of Pseudomonas aeruginosa type III secretion system.PLoS Pathog. 2022 Dec 5;18(12):e1011027. doi: 10.1371/journal.ppat.1011027. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36469533 Free PMC article.
-
Fitting Pieces into the Puzzle of Pseudomonas aeruginosa Type III Secretion System Gene Expression.J Bacteriol. 2019 Jun 10;201(13):e00209-19. doi: 10.1128/JB.00209-19. Print 2019 Jul 1. J Bacteriol. 2019. PMID: 31010903 Free PMC article. Review.
-
Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system.Mol Microbiol. 2006 Nov;62(3):631-40. doi: 10.1111/j.1365-2958.2006.05412.x. Epub 2006 Sep 21. Mol Microbiol. 2006. PMID: 16995895 Review.
Cited by
-
Global reprogramming of virulence and antibiotic resistance in Pseudomonas aeruginosa by a single nucleotide polymorphism in elongation factor, fusA1.J Biol Chem. 2020 Nov 27;295(48):16411-16426. doi: 10.1074/jbc.RA119.012102. Epub 2020 Sep 17. J Biol Chem. 2020. PMID: 32943550 Free PMC article.
-
The c-di-GMP Phosphodiesterase PipA (PA0285) Regulates Autoaggregation and Pf4 Bacteriophage Production in Pseudomonas aeruginosa PAO1.Appl Environ Microbiol. 2022 Jun 28;88(12):e0003922. doi: 10.1128/aem.00039-22. Epub 2022 May 31. Appl Environ Microbiol. 2022. PMID: 35638845 Free PMC article.
-
Cis-2-dodecenoic acid signal modulates virulence of Pseudomonas aeruginosa through interference with quorum sensing systems and T3SS.BMC Microbiol. 2013 Oct 18;13:231. doi: 10.1186/1471-2180-13-231. BMC Microbiol. 2013. PMID: 24134835 Free PMC article.
-
Substrate specificity and function of acetylpolyamine amidohydrolases from Pseudomonas aeruginosa.BMC Biochem. 2016 Mar 9;17:4. doi: 10.1186/s12858-016-0063-z. BMC Biochem. 2016. PMID: 26956223 Free PMC article.
-
Pan-genome dynamics of Pseudomonas gene complements enriched across hexachlorocyclohexane dumpsite.BMC Genomics. 2015 Apr 18;16(1):313. doi: 10.1186/s12864-015-1488-2. BMC Genomics. 2015. PMID: 25898829 Free PMC article.
References
-
- Galán JE, Bliska JB. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1996;12:221–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

