Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity
- PMID: 18074031
- PMCID: PMC2110898
- DOI: 10.1371/journal.pone.0001308
Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity
Abstract
Background: Probiotics are proposed to positively modulate the intestinal epithelial barrier formed by intestinal epithelial cells (IECs) and intercellular junctions. Disruption of this border alters paracellular permeability and is a key mechanism for the development of enteric infections and inflammatory bowel diseases (IBDs).
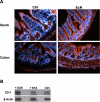
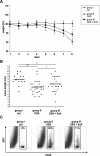
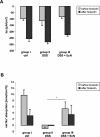
Methodology and principal findings: To study the in vivo effect of probiotic Escherichia coli Nissle 1917 (EcN) on the stabilization of the intestinal barrier under healthy conditions, germfree mice were colonized with EcN or K12 E. coli strain MG1655. IECs were isolated and analyzed for gene and protein expression of the tight junction molecules ZO-1 and ZO-2. Then, in order to analyze beneficial effects of EcN under inflammatory conditions, the probiotic was orally administered to BALB/c mice with acute dextran sodium sulfate (DSS) induced colitis. Colonization of gnotobiotic mice with EcN resulted in an up-regulation of ZO-1 in IECs at both mRNA and protein levels. EcN administration to DSS-treated mice reduced the loss of body weight and colon shortening. In addition, infiltration of the colon with leukocytes was ameliorated in EcN inoculated mice. Acute DSS colitis did not result in an anion secretory defect, but abrogated the sodium absorptive function of the mucosa. Additionally, intestinal barrier function was severely affected as evidenced by a strong increase in the mucosal uptake of Evans blue in vivo. Concomitant administration of EcN to DSS treated animals resulted in a significant protection against intestinal barrier dysfunction and IECs isolated from these mice exhibited a more pronounced expression of ZO-1.
Conclusion and significance: This study convincingly demonstrates that probiotic EcN is able to mediate up-regulation of ZO-1 expression in murine IECs and confer protection from the DSS colitis-associated increase in mucosal permeability to luminal substances.
Conflict of interest statement
Figures




References
-
- Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, et al. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991;81:327–334. - PubMed
-
- Rene E, Marche C, Regnier B, Saimot AG, Vilde JL, et al. Intestinal infections in patients with acquired immunodeficiency syndrome. A prospective study in 132 patients. Dig Dis Sci. 1989;34:773–780. - PubMed
-
- Smith PD, Lane HC, Gill VJ, Manischewitz JF, Quinnan GV, et al. Intestinal infections in patients with the acquired immunodeficiency syndrome (AIDS). Etiology and response to therapy. Ann Intern Med. 1988;108:328–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

