Abbreviated clinical study reports with investigational medicinal products for human use: current guidelines and recommendations
- PMID: 18074423
- PMCID: PMC2213811
- DOI: 10.3325/cmj.2007.6.871
Abbreviated clinical study reports with investigational medicinal products for human use: current guidelines and recommendations
Figures
References
-
- Molzon JA. The International Conference on Harmonization common technical document – global submission format? Food Drug Law J. 2005;60:447–51. - PubMed
-
- Todic M. Dossier for marketing authorization in the European union. Bosn J Basic Med Sci. 2003;3:56–60. - PubMed
-
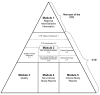
- CPMP/ICH. 2887/99. ICH M4E. Common technical document for the registration of pharmaceuticals for human use-efficacy. Clinical overview and clinical summary of module 2. Module 5: clinical study reports. London: European Medicines Agency; 2003.
-
- CPMP/ICH. 137/95. ICH topic E3. Structure and Content of Clinical Study Reports. London: European Medicines Agency; 1996.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical