The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age
- PMID: 18076286
- PMCID: PMC2121110
- DOI: 10.1371/journal.pbio.0050325
The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age
Abstract
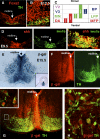
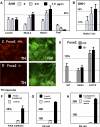
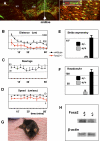
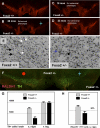
Parkinson disease affects more than 1% of the population over 60 y old. The dominant models for Parkinson disease are based on the use of chemical toxins to kill dopamine neurons, but do not address the risk factors that normally increase with age. Forkhead transcription factors are critical regulators of survival and longevity. The forkhead transcription factor, foxa2, is specifically expressed in adult dopamine neurons and their precursors in the medial floor plate. Gain- and loss-of-function experiments show this gene, foxa2, is required to generate dopamine neurons during fetal development and from embryonic stem cells. Mice carrying only one copy of the foxa2 gene show abnormalities in motor behavior in old age and an associated progressive loss of dopamine neurons. Manipulating forkhead function may regulate both the birth of dopamine neurons and their spontaneous death, two major goals of regenerative medicine.
Conflict of interest statement
Figures
Similar articles
-
Foxa2: the rise and fall of dopamine neurons.Cell Stem Cell. 2008 Feb 7;2(2):110-2. doi: 10.1016/j.stem.2008.01.012. Cell Stem Cell. 2008. PMID: 18371430 Review.
-
Foxa1 and Foxa2 transcription factors regulate differentiation of midbrain dopaminergic neurons.Adv Exp Med Biol. 2009;651:58-65. doi: 10.1007/978-1-4419-0322-8_5. Adv Exp Med Biol. 2009. PMID: 19731550 Review.
-
Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival.Stem Cells. 2010 Mar 31;28(3):501-12. doi: 10.1002/stem.294. Stem Cells. 2010. PMID: 20049900
-
Selection Based on FOXA2 Expression Is Not Sufficient to Enrich for Dopamine Neurons From Human Pluripotent Stem Cells.Stem Cells Transl Med. 2014 Sep;3(9):1032-42. doi: 10.5966/sctm.2014-0011. Epub 2014 Jul 14. Stem Cells Transl Med. 2014. PMID: 25024431 Free PMC article.
-
Foxa1 and Foxa2 orchestrate development of the urethral tube and division of the embryonic cloaca through an autoregulatory loop with Shh.Dev Biol. 2020 Sep 1;465(1):23-30. doi: 10.1016/j.ydbio.2020.06.009. Epub 2020 Jul 6. Dev Biol. 2020. PMID: 32645357 Free PMC article.
Cited by
-
Wnt5a cooperates with canonical Wnts to generate midbrain dopaminergic neurons in vivo and in stem cells.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):E602-10. doi: 10.1073/pnas.1208524110. Epub 2013 Jan 16. Proc Natl Acad Sci U S A. 2013. PMID: 23324743 Free PMC article.
-
Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance.Front Cell Neurosci. 2014 Sep 9;8:275. doi: 10.3389/fncel.2014.00275. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25249938 Free PMC article.
-
Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling.Cell Rep. 2014 Nov 6;9(3):930-43. doi: 10.1016/j.celrep.2014.10.008. Epub 2014 Oct 30. Cell Rep. 2014. PMID: 25437550 Free PMC article.
-
Parkinson's Disease Master Regulators on Substantia Nigra and Frontal Cortex and Their Use for Drug Repositioning.Mol Neurobiol. 2021 Apr;58(4):1517-1534. doi: 10.1007/s12035-020-02203-x. Epub 2020 Nov 19. Mol Neurobiol. 2021. PMID: 33211252
-
Toward Spatial Identities in Human Brain Organoids-on-Chip Induced by Morphogen-Soaked Beads.Bioengineering (Basel). 2020 Dec 18;7(4):164. doi: 10.3390/bioengineering7040164. Bioengineering (Basel). 2020. PMID: 33352983 Free PMC article.
References
-
- Lange KW, Paul GM, Robbins TW, Marsden CD. L-dopa and frontal cognitive function in Parkinson's disease. Adv Neurol. 1993;60:475–478. - PubMed
-
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, et al. Prevalence of Parkinson's Disease in Europe: a collaborative study of population-based cohorts. Neurology. 2000;54:S21–S23. - PubMed
-
- Altman J, Bayer S.A. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J Comp Neurol. 1981;198:677–716. - PubMed
-
- Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

