Leptin therapy for partial lipodystrophy linked to a PPAR-gamma mutation
- PMID: 18076675
- PMCID: PMC2578870
- DOI: 10.1111/j.1365-2265.2007.03095.x
Leptin therapy for partial lipodystrophy linked to a PPAR-gamma mutation
Abstract
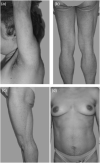
Aims/hypothesis: Partial lipodystrophy (PL) is most commonly characterized by loss of subcutaneous fat in the extremities with preservation of truncal fat and is associated with insulin resistance, diabetes and hyperlipidaemia. Recombinant human leptin (r-metHuLeptin) therapy has been shown to be effective in treating metabolic abnormalities associated with congenital or acquired generalized lipodystrophy and PL associated with lamin A/C (LMNA) gene mutations or highly active antiretroviral therapy (HAART). Our aim was to assess the effectiveness of leptin therapy in treating metabolic complications of PL associated with heterozygous peroxisome proliferator activated receptor gamma (PPARG) mutations. This is the first report to detail the clinical response of a patient with PL due to a PPARG mutation treated with r-metHuLeptin.
Methods: A 36-year-old female with PL associated with a heterozygous PPARG mutation complicated by poorly controlled diabetes and severe, refractory hypertriglyceridaemia was enrolled in a National Institutes of Health (NIH) protocol to evaluate the role of r-metHuLeptin in lipodystrophy. The patient received escalating doses of r-metHuLeptin until a dose 0.12 mg/kg/day was reached. Metabolic parameters, including serum chemistries, fasting blood glucose, glycated haemoglobin (HbA1c), lipid profile, an oral glucose tolerance test (OGTT), an insulin tolerance test (ITT), liver volume, percentage body fat and energy expenditure were followed at regular time intervals over 18 months of therapy.
Results: Eighteen months of r-MetHuLeptin therapy was associated with a marked improvement in glucose homeostasis as evidenced by normalization of the fasting blood glucose (baseline = 8.3 mmol/l; 18 months = 4.9 mmol/l), lowering of HbA1c (baseline = 9.9%; 18 months = 7.2%) and improved tolerance to an oral glucose load. In addition, a striking amelioration in the patient's refractory, severe hypertriglyceridaemia was observed (baseline = 21.15 mmol/l; 18 months = 5.96 mmol/l).
Conclusion: r-MetHuLeptin is effective in treating metabolic complications associated with PL due to PPARG mutations. In the context of previously published work, our findings suggest that the response to r-MetHuLeptin is independent of the aetiology in lipodystrophy.
Figures




References
-
- Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nature Genetics. 1998;18:292–295. - PubMed
-
- Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O'Rahilly S, Trembath RC. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nature Genetics. 2000;24:153–156. - PubMed
-
- Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Human Molecular Genetics. 2000;9:109–112. - PubMed
-
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. - PubMed
-
- Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. Journal of Clinical Endocrinology and Metabolism. 2002;87:408–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

