Selective activation of mechanosensitive ion channels using magnetic particles
- PMID: 18077244
- PMCID: PMC2495030
- DOI: 10.1098/rsif.2007.1274
Selective activation of mechanosensitive ion channels using magnetic particles
Abstract
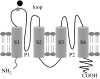
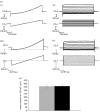
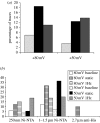
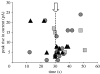
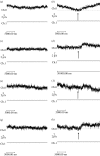
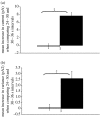
This study reports the preliminary development of a novel magnetic particle-based technique that permits the application of highly localized mechanical forces directly to specific regions of an ion-channel structure. We demonstrate that this approach can be used to directly and selectively activate a mechanosensitive ion channel of interest, namely TREK-1. It is shown that manipulation of particles targeted against the extended extracellular loop region of TREK-1 leads to changes in whole-cell currents consistent with changes in TREK-1 activity. Responses were absent when particles were coated with RGD (Arg-Gly-Asp) peptide or when magnetic fields were applied in the absence of magnetic particles. It is concluded that changes in whole-cell current are the result of direct force application to the extracellular loop region of TREK-1 and thus these results implicate this region of the channel structure in mechano-gating. It is hypothesized that the extended loop region of TREK-1 may act as a tension spring that acts to regulate sensitivity to mechanical forces, in a nature similar to that described for MscL. The development of a technique that permits the direct manipulation of mechanosensitive ion channels in real time without the need for pharmacological drugs has huge potential benefits not only for basic biological research of ion-channel gating mechanisms, but also potentially as a tool for the treatment of human diseases caused by ion-channel dysfunction.
Figures
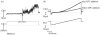
References
-
- Cartmell S, El Haj A.J. Mechanical bioreactors for tissue engineering. In: Chaudhuri J.B, Rubeai M.A, editors. Bioreactors for tissue engineering, principles, design and operation. Springer; Berlin, Germany: 2005. pp. 193–208. ch. 8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
