Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge
- PMID: 18077329
- PMCID: PMC2154454
- DOI: 10.1073/pnas.0710335105
Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge
Abstract
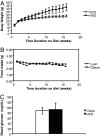
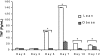
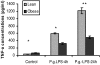
Obesity has been suggested to be associated with an increased susceptibility to bacterial infection. However, few studies have examined the effect of obesity on the immune response to bacterial infections. In the present study, we investigated the effect of obesity on innate immune responses to Porphyromonas gingivalis infection, an infection strongly associated with periodontitis. Mice with diet-induced obesity (DIO) and lean control C57BL/6 mice were infected orally or systemically with P. gingivalis, and periodontal pathology and systemic immune responses were examined postinfection. After oral infection with P. gingivalis, mice with DIO had a significantly higher level of alveolar bone loss than the lean controls. Oral microbial sampling disclosed higher levels of P. gingivalis in mice with DIO vs. lean mice during and after infection. Furthermore, animals with DIO exposed to oral infection or systemic inoculation of live P. gingivalis developed a blunted inflammatory response with reduced expression of TNF-alpha, IL-6, and serum amyloid A (SAA) at all time points compared with lean mice. Finally, peritoneal macrophages harvested from mice with DIO and exposed to P. gingivalis exhibited reduced levels of proinflammatory cytokines compared with lean mice and when exposed to P. gingivalis LPS treatment had a significantly reduced recruitment of NF-kappaB to both TNF-alpha and IL-10 promoters 30 min after exposure. These data indicate that obesity interferes with the ability of the immune system to appropriately respond to P. gingivalis infection and suggest that this immune dysregulation participates in the increased alveolar bone loss after bacterial infection observed in mice with DIO.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Dasanayake AP, et al. Preterm low birth weight and periodontal disease among African Americans. Dent Clin North Am. 2003;47:115–125. x–xi. - PubMed
-
- Nishimura F, et al. Periodontal infection and dyslipidemia in type 2 diabetics: Association with increased HMG-CoA reductase expression. Horm Metab Res. 2006;38:530–535. - PubMed
-
- Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and periodontal disease: A case-control study. J Periodontol. 2005;76:418–425. - PubMed
-
- Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: Pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

