Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode
- PMID: 18077369
- PMCID: PMC2154473
- DOI: 10.1073/pnas.0707574105
Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode
Abstract
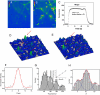
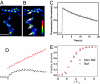
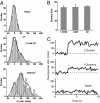
The nature of synaptic vesicle recycling at nerve terminals has been a subject of considerable debate for >35 years. Here, we report the use of an optical strategy that allows the exocytosis and retrieval of synaptic components to be tracked in real time at single-molecule sensitivity in living nerve terminals. This approach has allowed us to examine the recycling of synaptic vesicles in response to single action potentials. Our results show that, after exocytosis, individual synaptic vesicles are retrieved by a stochastic process with an exponential distribution of delay times, with a mean time of approximately 14 s. We propose that evidence for fast endocytosis, such as that proposed to support the presence of kiss-and-run, is likely explained by the stochastic nature of a slower process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
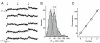
References
-
- Yamashita T, Hige T, Takahashi T. Science. 2005;307:124–127. - PubMed
-
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, et al. Science. 2007;316:570–574. - PubMed
-
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Neuron. 2006;51:773–786. - PubMed
-
- von Gersdorff H, Matthews G. Nature. 1994;367:735–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

