Dodecamer rotor ring defines H+/ATP ratio for ATP synthesis of prokaryotic V-ATPase from Thermus thermophilus
- PMID: 18077374
- PMCID: PMC2154418
- DOI: 10.1073/pnas.0706914105
Dodecamer rotor ring defines H+/ATP ratio for ATP synthesis of prokaryotic V-ATPase from Thermus thermophilus
Abstract
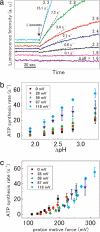
ATP synthesis by V-ATPase from the thermophilic bacterium Thermus thermophilus driven by the acid-base transition was investigated. The rate of ATP synthesis increased in parallel with the increase in proton motive force (PMF) >110 mV, which is composed of a difference in proton concentration (DeltapH) and the electrical potential differences (DeltaPsi) across membranes. The optimum rate of synthesis reached 85 s(-1), and the H(+)/ATP ratio of 4.0 +/- 0.1 was obtained. ATP was synthesized at a considerable rate solely by DeltapH, indicating DeltaPsi was not absolutely required for synthesis. Consistent with the H(+)/ATP ratio, cryoelectron micrograph images of 2D crystals of the membrane-bound rotor ring of the V-ATPase at 7.0-A resolution showed the presence of 12 V(o)-c subunits, each composed of two transmembrane helices. These results indicate that symmetry mismatch between the rotor and catalytic domains is not obligatory for rotary ATPases/synthases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

