Dynamic Stokes shift in green fluorescent protein variants
- PMID: 18077381
- PMCID: PMC2154406
- DOI: 10.1073/pnas.0706185104
Dynamic Stokes shift in green fluorescent protein variants
Abstract
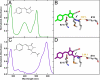
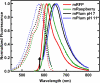
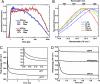
Solvent reorganization around the excited state of a chromophore leads to an emission shift to longer wavelengths during the excited-state lifetime. This solvation response is absent in wild-type green fluorescent protein, and this has been attributed to rigidity in the chromophore's environment necessary to exclude nonradiative transitions to the ground state. The fluorescent protein mPlum was developed via directed evolution by selection for red emission, and we use time-resolved fluorescence to study the dynamic Stokes shift through its evolutionary history. The far-red emission of mPlum is attributed to a picosecond solvation response that is observed at all temperatures above the glass transition. This time-dependent shift in emission is not observed in its evolutionary ancestors, suggesting that selective pressure has produced a chromophore environment that allows solvent reorganization. The evolutionary pathway and structures of related fluorescent proteins suggest the role of a single residue in close proximity to the chromophore as the primary cause of the solvation response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
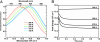
References
-
- Van der Zwan G. J Phys Chem. 1985;89:4181–4188.
-
- Horng ML, Gardecki JA, Papazyan A, Maroncelli M. J Phys Chem. 1995;99:17311–17337.
-
- Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA. Nat Biotech. 1999;17:969–973. - PubMed
-
- Homoelle BJ, Edington MD, Diffey WM, Beck WF. J Phys Chem B. 1998;102:3044–3052.
-
- Pierce D, Boxer SG. J Phys Chem. 1992;96:5560–5566.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

