Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2
- PMID: 18077391
- PMCID: PMC2409252
- DOI: 10.1073/pnas.0707394104
Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2
Abstract
Hematopoietic prostaglandin D(2) synthase (hPGD(2)S) metabolizes cyclooxygenase (COX)-derived PGH(2) to PGD(2) and 15-deoxyDelta(12-14) PGJ(2) (15d-PGJ(2)). Unlike COX, the role of hPGD(2)S in host defense is ambiguous. PGD(2) can be either pro- or antiinflammatory depending on disease etiology, whereas the existence of 15d-PGJ(2) and its relevance to pathophysiology remain controversial. Herein, studies on hPGD(2)S KO mice reveal that 15d-PGJ(2) is synthesized in a self-resolving peritonitis, detected by using liquid chromatography-tandem MS. Together with PGD(2) working on its DP1 receptor, 15d-PGJ(2) controls the balance of pro- vs. antiinflammatory cytokines that regulate leukocyte influx and monocyte-derived macrophage efflux from the inflamed peritoneal cavity to draining lymph nodes leading to resolution. Specifically, inflammation in hPGD(2)S KOs is more severe during the onset phase arising from a substantial cytokine imbalance resulting in enhanced polymorphonuclear leukocyte and monocyte trafficking. Moreover, resolution is impaired, characterized by macrophage and surprisingly lymphocyte accumulation. Data from this work place hPGD(2)S at the center of controlling the onset and the resolution of acute inflammation where it acts as a crucial checkpoint controller of cytokine/chemokine synthesis as well as leukocyte influx and efflux. Here, we provide definitive proof that 15d-PGJ(2) is synthesized during mammalian inflammatory responses, and we highlight DP1 receptor activation as a potential antiinflammatory strategy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



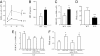
Comment in
-
Resolving the problem of persistence in the switch from acute to chronic inflammation.Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20647-8. doi: 10.1073/pnas.0710633105. Epub 2007 Dec 18. Proc Natl Acad Sci U S A. 2007. PMID: 18093938 Free PMC article. No abstract available.
References
-
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Nat Med. 1999;5:698–701. - PubMed
-
- Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. FASEB J. 2004;18:489–498. - PubMed
-
- Urade Y, Hayaishi O. Vitam Horm. 2000;58:89–120. - PubMed
-
- Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. J Immunol. 2003;171:3936–3940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

