Principles underlying energetic coupling along an allosteric communication trajectory of a voltage-activated K+ channel
- PMID: 18077413
- PMCID: PMC2148381
- DOI: 10.1073/pnas.0708120104
Principles underlying energetic coupling along an allosteric communication trajectory of a voltage-activated K+ channel
Abstract
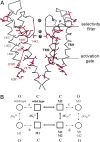
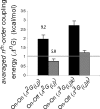
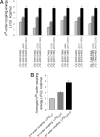
The information flow between distal elements of a protein may rely on allosteric communication trajectories lying along the protein's tertiary or quaternary structure. To unravel the underlying features of energy parsing along allosteric pathways in voltage-gated K(+) channels, high-order thermodynamic coupling analysis was performed. We report that such allosteric trajectories are functionally conserved and delineated by well defined boundaries. Moreover, allosteric trajectories assume a hierarchical organization whereby increasingly stronger layers of cooperative residue interactions act to ensure efficient and cooperative long-range coupling between distal channel regions. Such long-range communication is brought about by a coupling of local and global conformational changes, suggesting that the allosteric trajectory also corresponds to a pathway of physical deformation. Supported by theoretical analyses and analogy to studies analyzing the contribution of long-range residue coupling to protein stability, we propose that such experimentally derived trajectory features are a general property of allosterically regulated proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
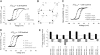
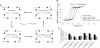
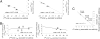
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

