Chorionic enhancer is dispensable for regulated expression of the human renin gene
- PMID: 18077515
- PMCID: PMC2408876
- DOI: 10.1152/ajpregu.00780.2007
Chorionic enhancer is dispensable for regulated expression of the human renin gene
Abstract
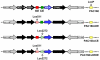
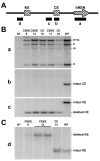
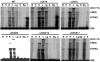
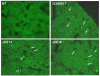
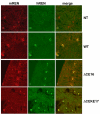
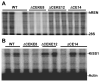
We tested the hypothesis that a transcriptional chorionic enhancer (CE), previously identified to increase human renin expression in choriodecidual cells is required to mediate tissue-specific, cell-specific, and regulated expression of human renin in transgenic mice. Recombineering was used to delete the CE upstream of the renin gene alone or in combination with the kidney enhancer (KE) in a large artificial chromosome construct containing the entire human renin gene and extensive flanking sequences. Deletion of the CE had no qualitative or quantitative effect on the tissue-specific expression of human renin, nor on the cellular localization of human renin in the kidney or placenta. Combined deletion of both the CE and KE caused a decrease in the level of renal renin expression consistent with the established role of the KE. We also considered the possibility that the CE is a downstream enhancer of the KiSS1 gene, which lies directly upstream of renin and is also expressed in the placenta. Deletion of the CE alone, or the CE and KE together, had no effect on the level of KiSS1 expression in the placenta. These data provide convincing evidence that the CE is silent in vivo, at least in the mouse. The absence of a phenotype caused by deletion of the CE is consistent with the observation that the sequence is not evolutionarily conserved.
Figures




Similar articles
-
Dysregulated human renin expression in transgenic mice carrying truncated genomic constructs: evidence supporting the presence of insulators at the renin locus.Am J Physiol Renal Physiol. 2008 Sep;295(3):F642-53. doi: 10.1152/ajprenal.00384.2007. Epub 2008 Jul 16. Am J Physiol Renal Physiol. 2008. PMID: 18632798 Free PMC article.
-
The human renin kidney enhancer is required to maintain base-line renin expression but is dispensable for tissue-specific, cell-specific, and regulated expression.J Biol Chem. 2006 Nov 17;281(46):35296-304. doi: 10.1074/jbc.M608055200. Epub 2006 Sep 21. J Biol Chem. 2006. PMID: 16990260
-
Conserved enhancer elements in human and mouse renin genes have different transcriptional effects in As4.1 cells.Circ Res. 1997 Oct;81(4):558-66. doi: 10.1161/01.res.81.4.558. Circ Res. 1997. PMID: 9314837
-
Transcriptional regulation of renin: an update.Hypertension. 2005 Jan;45(1):3-8. doi: 10.1161/01.HYP.0000149717.55920.45. Epub 2004 Nov 15. Hypertension. 2005. PMID: 15545507 Review.
-
Function of human renin proximal promoter DNA.Kidney Int. 1994 Dec;46(6):1516-21. doi: 10.1038/ki.1994.434. Kidney Int. 1994. PMID: 7699994 Review.
Cited by
-
Shadow enhancers: frequently asked questions about distributed cis-regulatory information and enhancer redundancy.Bioessays. 2012 Feb;34(2):135-41. doi: 10.1002/bies.201100121. Epub 2011 Nov 15. Bioessays. 2012. PMID: 22083793 Free PMC article.
-
Classical Renin-Angiotensin system in kidney physiology.Compr Physiol. 2014 Jul;4(3):1201-28. doi: 10.1002/cphy.c130040. Compr Physiol. 2014. PMID: 24944035 Free PMC article. Review.
-
Control of renin [corrected] gene expression.Pflugers Arch. 2013 Jan;465(1):13-21. doi: 10.1007/s00424-012-1110-2. Epub 2012 May 11. Pflugers Arch. 2013. PMID: 22576577 Free PMC article. Review.
-
Dysregulated human renin expression in transgenic mice carrying truncated genomic constructs: evidence supporting the presence of insulators at the renin locus.Am J Physiol Renal Physiol. 2008 Sep;295(3):F642-53. doi: 10.1152/ajprenal.00384.2007. Epub 2008 Jul 16. Am J Physiol Renal Physiol. 2008. PMID: 18632798 Free PMC article.
-
Enhancer redundancy in development and disease.Nat Rev Genet. 2021 May;22(5):324-336. doi: 10.1038/s41576-020-00311-x. Epub 2021 Jan 12. Nat Rev Genet. 2021. PMID: 33442000 Free PMC article. Review.
References
-
- Adams DJ, Head GA, Markus MA, Lovicu FJ, van der WL, Kontgen F, Arends MJ, Thiru S, Mayorov DN, Morris BJ. Renin enhancer is critical for control of renin gene expression and cardiovascular function. J Biol Chem. 2006;281:31753–31761. - PubMed
-
- Baumann H, Wang Y, Richards CD, Jones CA, Black TA, Gross KW. Endotoxin-induced renal inflammatory response. Oncostatin M as a major mediator of suppressed renin expression. J Biol Chem. 2000;275:22014–22019. - PubMed
-
- Fuchs S, Philippe J, Germain S, Mathieu F, Jeunemaitre X, Corvol P, Pinet F. Functionality of two new polymorphisms in the human renin gene enhancer region. J Hypertens. 2002;20:2391–2398. - PubMed
-
- Germain S, Bonnet F, Philippe J, Fuchs S, Corvol P, Pinet F. A novel distal enhancer confers chorionic expression on the human renin gene. J Biol Chem. 1998;273:25292–25300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

