Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIalpha
- PMID: 18077555
- PMCID: PMC2230591
- DOI: 10.1091/mbc.e07-07-0713
Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIalpha
Abstract
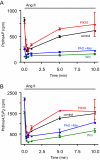
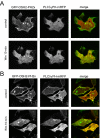
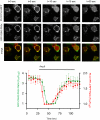
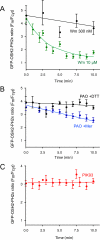
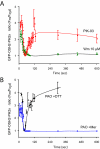
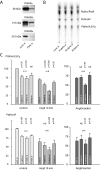
Type III phosphatidylinositol (PtdIns) 4-kinases (PI4Ks) have been previously shown to support plasma membrane phosphoinositide synthesis during phospholipase C activation and Ca(2+) signaling. Here, we use biochemical and imaging tools to monitor phosphoinositide changes in the plasma membrane in combination with pharmacological and genetic approaches to determine which of the type III PI4Ks (alpha or beta) is responsible for supplying phosphoinositides during agonist-induced Ca(2+) signaling. Using inhibitors that discriminate between the alpha- and beta-isoforms of type III PI4Ks, PI4KIIIalpha was found indispensable for the production of phosphatidylinositol 4-phosphate (PtdIns4P), phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P(2)], and Ca(2+) signaling in angiotensin II (AngII)-stimulated cells. Down-regulation of either the type II or type III PI4K enzymes by small interfering RNA (siRNA) had small but significant effects on basal PtdIns4P and PtdIns(4,5)P(2) levels in (32)P-labeled cells, but only PI4KIIIalpha down-regulation caused a slight impairment of PtdIns4P and PtdIns(4,5)P(2) resynthesis in AngII-stimulated cells. None of the PI4K siRNA treatments had a measurable effect on AngII-induced Ca(2+) signaling. These results indicate that a small fraction of the cellular PI4K activity is sufficient to maintain plasma membrane phosphoinositide pools, and they demonstrate the value of the pharmacological approach in revealing the pivotal role of PI4KIIIalpha enzyme in maintaining plasma membrane phosphoinositides.
Figures



References
-
- Audhya A., Emr S. D. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2002;2:593–605. - PubMed
-
- Balla A., Balla T. Phosphatidylinositol 4-kinases; old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. - PubMed
-
- Balla A., Tuymetova G., Barshishat M., Geiszt M., Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 2002;277:20041–22050. - PubMed
-
- Balla A., Tuymetova G., Tsiomenko A., Varnai P., Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell. 2005;16:1282–1295. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

