Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons
- PMID: 18077664
- PMCID: PMC2692874
- DOI: 10.1152/jn.00930.2007
Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons
Abstract
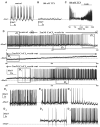
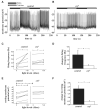
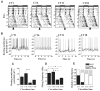
The ventral lateral neurons (LNvs) of adult Drosophila brain express oscillating clock proteins and regulate circadian behavior. Whole cell current-clamp recordings of large LNvs in freshly dissected Drosophila whole brain preparations reveal two spontaneous activity patterns that correlate with two underlying patterns of oscillating membrane potential: tonic and burst firing of sodium-dependent action potentials. Resting membrane potential and spontaneous action potential firing are rapidly and reversibly regulated by acute changes in light intensity. The LNv electrophysiological light response is attenuated, but not abolished, in cry(b) mutant flies hypomorphic for the cell-autonomous light-sensing protein CRYPTOCHROME. The electrical activity of the large LNv is circadian regulated, as shown by significantly higher resting membrane potential and frequency of spontaneous action potential firing rate and burst firing pattern during circadian subjective day relative to subjective night. The circadian regulation of membrane potential, spontaneous action potential firing frequency, and pattern of Drosophila large LNvs closely resemble mammalian circadian neuron electrical characteristics, suggesting a general evolutionary conservation of both physiological and molecular oscillator mechanisms in pacemaker neurons.
Figures

References
-
- Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr Biol. 2006;16:441–449. - PubMed
-
- de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725–3729. - PubMed
-
- de Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol. 2002;87:834–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

