Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington's disease
- PMID: 18077673
- PMCID: PMC6673605
- DOI: 10.1523/JNEUROSCI.3455-07.2007
Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington's disease
Abstract
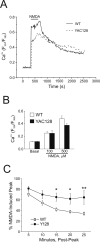
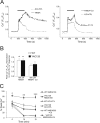
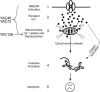
Expansion of a CAG repeat in the Huntington's disease (HD) gene results in progressive neuronal loss, particularly of striatal medium-sized spiny neurons (MSNs). Studies in human HD autopsy brain tissue, as well as cellular and animal models of HD, suggest that increased activity of NMDA-type glutamate receptors and altered mitochondrial function contribute to selective neuronal degeneration. In this regard, the YAC128 mouse model, expressing full-length human huntingtin with 128 glutamine repeats, has been the focus of much interest. Although NMDA-induced apoptosis is enhanced in YAC128 MSNs, here we report that the initial steps in the death signaling pathway, including NMDA receptor (NMDAR) current and cytosolic Ca2+ loading, are similar to those observed in wild-type MSNs. In contrast, we found that the NMDAR-mediated Ca2+ load triggered a strikingly enhanced loss of mitochondrial membrane potential in YAC128 MSNs, suggesting that NMDAR signaling via the mitochondrial apoptotic pathway is altered. This effect was accompanied by impaired cytosolic Ca2+ clearance after removal of NMDA, a difference that was not apparent after high potassium-evoked depolarization-mediated Ca2+ entry. Inhibition of the mitochondrial permeability transition (mPT) reduced peak cytosolic Ca2+ and mitochondrial depolarization evoked by NMDA in YAC128 MSNs but not wild-type MSNs. Hence, in contrast to YAC models with moderate CAG expansions, the enhanced NMDA-induced apoptosis in YAC128 MSNs is predominantly determined by augmented mitochondrial sensitivity to Ca2+-induced activation of the mPT. These results suggest that the CAG repeat length influences the mechanism by which mHtt enhances NMDAR-mediated excitotoxicity.
Figures



References
-
- Abdel-Hamid KM, Baimbridge KG. The effects of artificial calcium buffers on calcium responses and glutamate-mediated excitotoxicity in cultured hippocampal neurons. Neuroscience. 1997;81:673–687. - PubMed
-
- Alano CC, Beutner G, Dirksen RT, Gross RA, Sheu SS. Mitochondrial permeability transition and calcium dynamics in striatal neurons upon intense NMDA receptor activation. J Neurochem. 80:531–538. - PubMed
-
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. - PubMed
-
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. - PubMed
-
- Bittenbender JB, Quadfasel FA. Rigid and akinetic forms of Huntington's chorea. Arch Neurol. 1962;7:275–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
