The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning
- PMID: 18077674
- PMCID: PMC6673626
- DOI: 10.1523/JNEUROSCI.2858-07.2007
The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning
Abstract
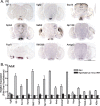
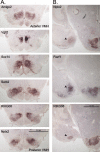
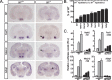
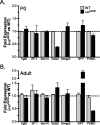
The ventromedial hypothalamus (VMH) is a distinct morphological nucleus involved in feeding, fear, thermoregulation, and sexual activity. It is essentially unknown how VMH circuits underlying these innate responses develop, in part because the VMH remains poorly defined at a cellular and molecular level. Specifically, there is a paucity of cell-type-specific genetic markers with which to identify neuronal subgroups and manipulate development and signaling in vivo. Using gene profiling, we now identify approximately 200 genes highly enriched in neonatal (postnatal day 0) mouse VMH tissue. Analyses of these VMH markers by real or virtual (Allen Brain Atlas; http://www.brain-map.org) experiments revealed distinct regional patterning within the newly formed VMH. Top neonatal markers include transcriptional regulators such as Vgll2, SF-1, Sox14, Satb2, Fezf1, Dax1, Nkx2-2, and COUP-TFII, but interestingly, the highest expressed VMH transcript, the transcriptional coregulator Vgll2, is completely absent in older animals. Collective results from zebrafish knockdown experiments and from cellular studies suggest that a subset of these VMH markers will be important for hypothalamic development and will be downstream of SF-1, a critical factor for normal VMH differentiation. We show that at least one VMH marker, the AT-rich binding protein Satb2, was responsive to the loss of leptin signaling (Lep(ob/ob)) at postnatal day 0 but not in the adult, suggesting that some VMH transcriptional programs might be influenced by fetal or early postnatal environments. Our study describing this comprehensive "VMH transcriptome" provides a novel molecular toolkit to probe further the genetic basis of innate neuroendocrine behavioral responses.
Figures


References
-
- Altman J, Bayer SA. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol. 1986;100:1–178. - PubMed
-
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. - PubMed
-
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. - PubMed
-
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. - PubMed
-
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
