Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms
- PMID: 18077675
- PMCID: PMC6673606
- DOI: 10.1523/JNEUROSCI.3949-07.2007
Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms
Abstract
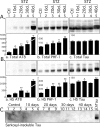
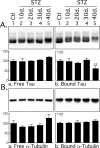
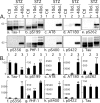
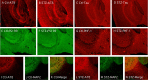
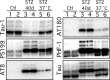
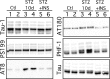
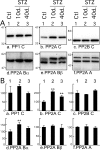
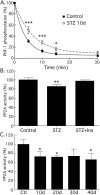
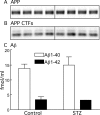
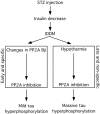
Hyperphosphorylated tau is the major component of paired helical filaments in neurofibrillary tangles found in Alzheimer's disease (AD) brains, and tau hyperphosphorylation is thought to be a critical event in the pathogenesis of the disease. The large majority of AD cases is late onset and sporadic in origin, with aging as the most important risk factor. Insulin resistance, impaired glucose tolerance, and diabetes mellitus (DM) are other common syndromes in the elderly also strongly age dependent, and there is evidence supporting a link between insulin dysfunction and AD. To investigate the possibility that insulin dysfunction might promote tau pathology, we induced insulin deficiency and caused DM in mice with streptozotocin (STZ). A mild hyperphosphorylation of tau could be detected 10, 20, and 30 d after STZ injection, and a massive hyperphosphorylation of tau was observed after 40 d. The robust hyperphosphorylation of tau was localized in the axons and neuropil, and prevented tau binding to microtubules. Neither mild nor massive tau phosphorylation induced tau aggregation. Body temperature of the STZ-treated mice did not differ from control animals during 30 d, but dropped significantly thereafter. No change in beta-amyloid (Abeta) precursor protein (APP), APP C-terminal fragments, or Abeta levels were observed in STZ-treated mice; however, cellular protein phosphatase 2A activity was significantly decreased. Together, these data indicate that insulin dysfunction induced abnormal tau hyperphosphorylation through two distinct mechanisms: one was consequent to hypothermia; the other was temperature-independent, inherent to insulin depletion, and probably caused by inhibition of phosphatase activity.
Figures


Similar articles
-
Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease.J Neurosci. 2004 Mar 10;24(10):2401-11. doi: 10.1523/JNEUROSCI.5561-03.2004. J Neurosci. 2004. PMID: 15014115 Free PMC article.
-
Insulin deprivation induces PP2A inhibition and tau hyperphosphorylation in hTau mice, a model of Alzheimer's disease-like tau pathology.Sci Rep. 2017 Apr 12;7:46359. doi: 10.1038/srep46359. Sci Rep. 2017. PMID: 28402338 Free PMC article.
-
Tau hyperphosphorylation in the brain of ob/ob mice is due to hypothermia: Importance of thermoregulation in linking diabetes and Alzheimer's disease.Neurobiol Dis. 2017 Feb;98:1-8. doi: 10.1016/j.nbd.2016.10.004. Epub 2016 Oct 26. Neurobiol Dis. 2017. PMID: 27793638
-
Insulin dysfunction and Tau pathology.Front Cell Neurosci. 2014 Feb 11;8:22. doi: 10.3389/fncel.2014.00022. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24574966 Free PMC article. Review.
-
Diabetes mellitus and Alzheimer's disease: GSK-3β as a potential link.Behav Brain Res. 2018 Feb 26;339:57-65. doi: 10.1016/j.bbr.2017.11.015. Epub 2017 Nov 21. Behav Brain Res. 2018. PMID: 29158110 Review.
Cited by
-
Deregulation of protein phosphatase 2A and hyperphosphorylation of τ protein following onset of diabetes in NOD mice.Diabetes. 2013 Feb;62(2):609-17. doi: 10.2337/db12-0187. Epub 2012 Sep 6. Diabetes. 2013. PMID: 22961084 Free PMC article.
-
Temperature-induced Artifacts in Tau Phosphorylation: Implications for Reliable Alzheimer's Disease Research.Exp Neurobiol. 2023 Dec 31;32(6):423-440. doi: 10.5607/en23025. Exp Neurobiol. 2023. PMID: 38196137 Free PMC article.
-
Diabetes Mellitus Induces Alzheimer's Disease Pathology: Histopathological Evidence from Animal Models.Int J Mol Sci. 2016 Apr 5;17(4):503. doi: 10.3390/ijms17040503. Int J Mol Sci. 2016. PMID: 27058526 Free PMC article. Review.
-
Cognitive deficits and Alzheimer-like neuropathological impairments during adolescence in a rat model of type 2 diabetes mellitus.Neural Regen Res. 2018 Nov;13(11):1995-2004. doi: 10.4103/1673-5374.239448. Neural Regen Res. 2018. PMID: 30233075 Free PMC article.
-
Transcriptomic analysis of diabetic kidney disease and neuropathy in mouse models of type 1 and type 2 diabetes.Dis Model Mech. 2023 Oct 1;16(10):dmm050080. doi: 10.1242/dmm.050080. Epub 2023 Oct 4. Dis Model Mech. 2023. PMID: 37791586 Free PMC article.
References
-
- Alonso AC, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–34881. - PubMed
-
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
-
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
