NR2A-/- mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus
- PMID: 18077676
- PMCID: PMC6673614
- DOI: 10.1523/JNEUROSCI.3153-07.2007
NR2A-/- mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus
Abstract
Whether the subunit composition of NMDA receptors (NMDARs) controls the direction of long-term plasticity is currently disputed. In the visual layers of NR2A-/- juvenile superior colliculus (SC), synapses lose miniature NMDAR currents, leaving NR2B-rich receptors in extrasynaptic regions. Compared with wild type (WT), evoked NMDAR currents in mutant neurons have slower rise and decay times and lower NMDAR/AMPAR current ratios. Moreover, NMDAR and L-type Ca2+ channel-dependent SC long-term potentiation (LTP) is absent in NR2A-/- cells, whereas both WT and mutant neurons show long-duration, low-frequency-induced, long-term depression (LLF-LTD) that is blocked by either AP-5, nimodipine, or Ro 25-6981 [R-(R,S)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidine propranol]. Thus, NMDAR currents or signaling localized at the postsynaptic density are essential to SC NMDAR-dependent LTP, whereas extrasynaptic or NR2B-rich NMDARs are necessary for LLF-LTD. However, synaptic NMDARs as well as the NR2A subunit are missing in NR2A-/- mice. Therefore, NR2 subunit-specific ligand binding/channel properties and/or separate signaling pathways interacting with NMDARs at synaptic versus extrasynaptic receptors could underlie these results.
Figures



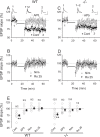
References
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Cavus I, Teyler T. Two forms of long-term potentiation in area CA1 activate different signal transduction cascades. J Neurophysiol. 1996;76:3038–3047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
