TRPM8 mechanism of cold allodynia after chronic nerve injury
- PMID: 18077679
- PMCID: PMC6673615
- DOI: 10.1523/JNEUROSCI.2203-07.2007
TRPM8 mechanism of cold allodynia after chronic nerve injury
Abstract
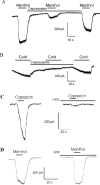
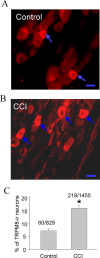
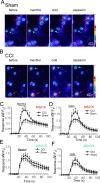
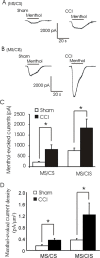
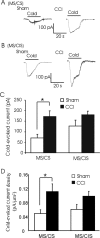
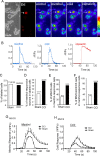
The cold- and menthol-sensitive receptor TRPM8 (transient receptor potential melastatin 8) has been suggested to play a role in cold allodynia, an intractable pain seen clinically. We studied how TRPM8 is involved in cold allodynia using rats with chronic constrictive nerve injury (CCI), a neuropathic pain model manifesting cold allodynia in hindlimbs. We found that cold allodynic response in the CCI animals was significantly attenuated by capsazepine, a blocker for both TRPM8 and TRPV1 (transient receptor potential vanilloid 1) receptors, but not by the selective TRPV1 antagonist I-RTX (5-iodoresiniferatoxin). In L5 dorsal root ganglion (DRG) sections of the CCI rats, immunostaining showed an increase in the percentage of TRPM8-immunoreactive neurons when compared with the sham group. Using the Ca2+-imaging technique and neurons acutely dissociated from the L5 DRGs, we found that CCI resulted in a significant increase in the percentage of menthol- and cold-sensitive neurons and also a substantial enhancement in the responsiveness of these neurons to both menthol and innocuous cold. These changes occurred in capsaicin-sensitive neurons, a subpopulation of nociceptive-like neurons. Using patch-clamp recordings, we found that membrane currents evoked by both menthol and innocuous cold were significantly enhanced in the CCI group compared with the sham group. By retrograde labeling afferent neurons that target hindlimb skin, we showed that the skin neurons expressed TRPM8 receptors, that the percentage of menthol-sensitive/cold-sensitive/capsaicin-sensitive neurons increased, and that the menthol- and cold-evoked responses were significantly enhanced in capsaicin-sensitive neurons after CCI. Together, the gain of TRPM8-mediated cold sensitivity on nociceptive afferent neurons provides a mechanism of cold allodynia.
Figures


References
-
- Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98. - PubMed
-
- Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca(2+)-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett. 2006;397:140–144. - PubMed
-
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
