Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease
- PMID: 18077684
- PMCID: PMC6673629
- DOI: 10.1523/JNEUROSCI.3379-07.2007
Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease
Abstract
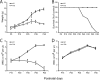
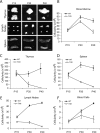
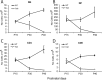
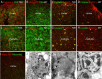
Lysosomal beta-galactosylceramidase deficiency results in demyelination and inflammation in the nervous system causing the neurological Krabbe disease. In the Twitcher mouse model of this disease, we found that neurological symptoms parallel progressive and severe lymphopenia. Although lymphopoiesis is normal before disease onset, primary and secondary lymphoid organs progressively degenerate afterward. This occurs despite preserved erythropoiesis and leads to severe peripheral lymphopenia caused by reduced numbers of T cell precursors and mature lymphocytes. Hematopoietic cell replacement experiments support the existence of an epigenetic factor in mutant mice reconcilable with a progressive loss of autonomic axons that hampers thymic functionality. We propose that degeneration of autonomic nerves leads to the irreversible thymic atrophy and loss of immune-competence. Our study describes a new aspect of Krabbe disease, placing patients at risk of immune-related pathologies, and identifies a novel target for therapeutic interventions.
Figures



References
-
- Antonica A, Ayroldi E, Magni F, Paolocci N. Lymphocyte traffic changes induced by monolateral vagal denervation in mouse thymus and peripheral lymphoid organs. J Neuroimmunol. 1996;64:115–122. - PubMed
-
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Bullard W, Southard E. Difuse gliosis of the cerebral white matter in a child. J Nerv Ment Dis. 1906;33:188–193.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
